Increased immunogenicity of avian influenza DNA vaccine delivered to the skin using a microneedle patch
- PMID: 22504442
- PMCID: PMC3362687
- DOI: 10.1016/j.ejpb.2012.03.010
Increased immunogenicity of avian influenza DNA vaccine delivered to the skin using a microneedle patch
Abstract
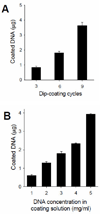
Effective public health responses to an influenza pandemic require an effective vaccine that can be manufactured and administered to large populations in the shortest possible time. In this study, we evaluated a method for vaccination against avian influenza virus that uses a DNA vaccine for rapid manufacturing and delivered by a microneedle skin patch for simplified administration and increased immunogenicity. We prepared patches containing 700-μm long microneedles coated with an avian H5 influenza hemagglutinin DNA vaccine from A/Viet Nam/1203/04 influenza virus. The coating DNA dose increased with DNA concentration in the coating solution and the number of dip-coating cycles. Coated DNA was released into the skin tissue by dissolution within minutes. Vaccination of mice using microneedles induced higher levels of antibody responses and hemagglutination inhibition titers, and improved protection against lethal infection with avian influenza as compared to conventional intramuscular delivery of the same dose of the DNA vaccine. Additional analysis showed that the microneedle coating solution containing carboxymethylcellulose and a surfactant may have negatively affected the immunogenicity of the DNA vaccine. Overall, this study shows that DNA vaccine delivery by microneedles can be a promising approach for improved vaccination to mitigate an influenza pandemic.
Copyright © 2012 Elsevier B.V. All rights reserved.
Conflict of interest statement
This potential conflict of interest has been disclosed and is being managed by Georgia Tech and Emory University.
Figures



References
-
- Monto AS. The threat of an avian influenza pandemic. N.Engl.J.Med. 2005;352:323–325. - PubMed
-
- Robertson JS, Nicolson C, Harvey R, Johnson R, Major D, Guilfoyle K, Roseby S, Newman R, Collin R, Wallis C, Engelhardt OG, Wood JM, Le JH, Manojkumar R, Pokorny BA, Silverman J, Devis R, Bucher D, Verity E, Agius C, Camuglia S, Ong C, Rockman S, Curtis A, Schoofs P, Zoueva O, Xie H, Li X, Lin ZS, Ye ZP, Chen LM, O'Neill E, Balishe A, Lipatov AS, Guo Z, Isakova I, Davis CT, Rivailler P, Gustin KM, Belser JA, Maines TR, Tumpey TM, Xu XY, Katz JM, Klimov A, Cox NJ, Donis RO. The development of vaccine viruses against pandemic A(H1N1) influenza. Vaccine. 2011;29:1836–1843. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

