Diversity of Listeria species in urban and natural environments
- PMID: 22504820
- PMCID: PMC3370529
- DOI: 10.1128/AEM.00282-12
Diversity of Listeria species in urban and natural environments
Abstract
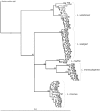
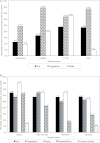
A total of 442 Listeria isolates, including 234 Listeria seeligeri, 80 L. monocytogenes, 74 L. welshimeri, 50 L. innocua, and 4 L. marthii isolates, were obtained from 1,805 soil, water, and other environmental samples collected over 2 years from four urban areas and four areas representing natural environments. Listeria spp. showed similar prevalences in samples from natural (23.4%) and urban (22.3%) environments. While L. seeligeri and L. welshimeri were significantly associated with natural environments (P ≤ 0.0001), L. innocua and L. monocytogenes were significantly associated with urban environments (P ≤ 0.0001). Sequencing of sigB for all isolates revealed 67 allelic types with a higher level of allelic diversity among isolates from urban environments. Some Listeria spp. and sigB allelic types showed significant associations with specific urban and natural areas. Nearest-neighbor analyses also showed that certain Listeria spp. and sigB allelic types were spatially clustered within both natural and urban environments, and there was evidence that these species and allelic types persisted over time in specific areas. Our data show that members of the genus Listeria not only are common in urban and natural environments but also show species- and subtype-specific associations with different environments and areas. This indicates that Listeria species and subtypes within these species may show distinct ecological preferences, which suggests (i) that molecular source-tracking approaches can be developed for Listeria and (ii) that detection of some Listeria species may not be a good indicator for L. monocytogenes.
Figures



References
-
- Bauwens L, Vercammen F, De Meurichy W. 2001. Occurrence of Listeria spp. in captive antelope herds and their environment. J. Zoo Wildl. Med. 32:514–518 - PubMed
-
- Bernagozzi M, Bianucci F, Sacchetti R, Bisbini P. 1994. Study of the prevalence of Listeria spp. in surface water. Zentralbl. Hyg. Umweltmed. 196:237–244 - PubMed
-
- Boerlin P, Rocourt J, Piffaretti JC. 1991. Taxonomy of the genus Listeria by using multilocus enzyme electrophoresis. Int. J. Syst. Bacteriol. 41:59–64 - PubMed
-
- Bouttefroy A, Lemaitre JP, Rousset A. 1997. Prevalence of Listeria sp. in droppings from urban rooks (Corvus frugilegus). J. Appl. Microbiol. 82:641–647 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

