Crystallization and preliminary X-ray diffraction analysis of human endoplasmic reticulum aminopeptidase 2
- PMID: 22505422
- PMCID: PMC3325822
- DOI: 10.1107/S1744309112006963
Crystallization and preliminary X-ray diffraction analysis of human endoplasmic reticulum aminopeptidase 2
Abstract
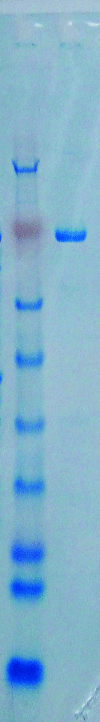
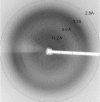
Endoplasmic reticulum aminopeptidase 2 (ERAP2) is a critical enzyme involved in the final processing of MHC class I antigens. Peptide trimming by ERAP2 and the other members of the oxytocinase subfamily is essential to customize longer precursor peptides in order to fit them to the correct length required for presentation on major histocompatibility complex class I molecules. While recent structures of ERAP1 have provided an understanding of the `molecular-ruler' mechanism of substrate selection, little is known about the complementary activities of its homologue ERAP2 despite their sharing 49% sequence identity. In order to gain insights into the structure-function relationship of the oxytocinase subfamily, and in particular ERAP2, the luminal region of human ERAP2 has been crystallized in the presence of the inhibitor bestatin. The crystals belonged to an orthorhombic space group and diffracted anisotropically to 3.3 Å resolution in the best direction on an in-house X-ray source. A molecular-replacement solution suggested that the enzyme has adopted the closed state as has been observed in other inhibitor-bound aminopeptidase structures.
© 2012 International Union of Crystallography. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

