The DC receptor DNGR-1 mediates cross-priming of CTLs during vaccinia virus infection in mice
- PMID: 22505455
- PMCID: PMC3336985
- DOI: 10.1172/JCI60660
The DC receptor DNGR-1 mediates cross-priming of CTLs during vaccinia virus infection in mice
Abstract
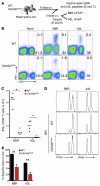
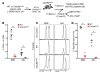
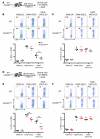
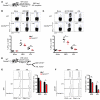
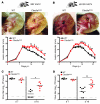
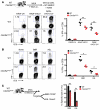
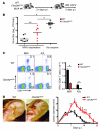
In order to prime T cells, DCs integrate signals emanating directly from pathogens and from their noxious action on the host. DNGR-1 (CLEC9A) is a DC-restricted receptor that detects dead cells. Therefore, we investigated the possibility that DNGR-1 affects immunity to cytopathic viruses. DNGR-1 was essential for cross-presentation of dying vaccinia virus-infected (VACV-infected) cells to CD8(+) T cells in vitro. Following injection of VACV or VACV-infected cells into mice, DNGR-1 detected the ligand in dying infected cells and mediated cross-priming of anti-VACV CD8(+) T cells. Loss of DNGR-1 impaired the CD8+ cytotoxic response to VACV, especially against those virus strains that are most dependent on cross-presentation. The decrease in total anti-VACV CTL activity was associated with a profound increase in viral load and delayed resolution of the primary lesion. In addition, lack of DNGR-1 markedly diminished protection from infection induced by vaccination with the modified vaccinia Ankara (MVA) strain. DNGR-1 thus contributes to anti-VACV immunity, following both primary infection and vaccination. The non-redundant ability of DNGR-1 to regulate cross-presentation of viral antigens suggests that this form of regulation of antiviral immunity could be exploited for vaccination.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

