The dendritic cell receptor DNGR-1 controls endocytic handling of necrotic cell antigens to favor cross-priming of CTLs in virus-infected mice
- PMID: 22505458
- PMCID: PMC3336984
- DOI: 10.1172/JCI60644
The dendritic cell receptor DNGR-1 controls endocytic handling of necrotic cell antigens to favor cross-priming of CTLs in virus-infected mice
Abstract
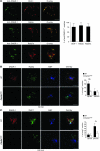
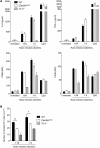
DNGR-1 (CLEC9A) is a receptor for necrotic cells required by DCs to cross-prime CTLs against dead cell antigens in mice. It is currently unknown how DNGR-1 couples dead cell recognition to cross-priming. Here we found that DNGR-1 did not mediate DC activation by dead cells but rather diverted necrotic cell cargo into a recycling endosomal compartment, favoring cross-presentation to CD8(+) T cells. DNGR-1 regulated cross-priming in non-infectious settings such as immunization with antigen-bearing dead cells, as well as in highly immunogenic situations such as infection with herpes simplex virus type 1. Together, these results suggest that DNGR-1 is a dedicated receptor for cross-presentation of cell-associated antigens. Our work thus underscores the importance of cross-priming in immunity and indicates that antigenicity and adjuvanticity can be decoded by distinct innate immune receptors. The identification of specialized receptors that regulate antigenicity of virus-infected cells reveals determinants of antiviral immunity that might underlie the human response to infection and vaccination.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

