Kallikrein 6 is a novel molecular trigger of reactive astrogliosis
- PMID: 22505518
- PMCID: PMC3335747
- DOI: 10.1515/hsz-2011-0241
Kallikrein 6 is a novel molecular trigger of reactive astrogliosis
Abstract
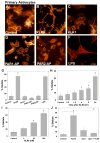
Kallikrein-related peptidase 6 (KLK6) is a trypsin-like serine protease upregulated at sites of central nervous system (CNS) injury, including de novo expression by reactive astrocytes, yet its physiological actions are largely undefined. Taken with recent evidence that KLK6 activates G-protein-coupled protease-activated receptors (PARs), we hypothesized that injury-induced elevations in KLK6 contribute to the development of astrogliosis and that this occurs in a PAR-dependent fashion. Using primary murine astrocytes and the Neu7 astrocyte cell line, we show that KLK6 causes astrocytes to transform from an epitheliod to a stellate morphology and to secrete interleukin 6 (IL-6). By contrast, KLK6 reduced expression of glial fibrillary acidic protein (GFAP). The stellation-promoting activities of KLK6 were shown to be dependent on activation of the thrombin receptor, PAR1, as a PAR1-specific inhibitor, SCH79797, blocked KLK6-induced morphological changes. The ability of KLK6 to promote astrocyte stellation was also shown to be linked to activation of protein kinase C (PKC). These studies indicate that KLK6 is positioned to serve as a molecular trigger of select physiological processes involved in the development of astrogliosis and that this is likely to occur at least in part by activation of the G-protein-coupled receptor, PAR1.
Figures


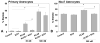

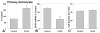
Similar articles
-
Critical role for PAR1 in kallikrein 6-mediated oligodendrogliopathy.Glia. 2013 Sep;61(9):1456-70. doi: 10.1002/glia.22534. Epub 2013 Jul 8. Glia. 2013. PMID: 23832758 Free PMC article.
-
Protease-activated receptor dependent and independent signaling by kallikreins 1 and 6 in CNS neuron and astroglial cell lines.J Neurochem. 2008 Nov;107(3):855-70. doi: 10.1111/j.1471-4159.2008.05658.x. Epub 2008 Sep 6. J Neurochem. 2008. PMID: 18778305 Free PMC article.
-
Kallikrein 6 signals through PAR1 and PAR2 to promote neuron injury and exacerbate glutamate neurotoxicity.J Neurochem. 2013 Oct;127(2):283-98. doi: 10.1111/jnc.12293. Epub 2013 May 27. J Neurochem. 2013. PMID: 23647384 Free PMC article.
-
Heterogeneity of reactive astrocytes.Neurosci Lett. 2014 Apr 17;565:23-9. doi: 10.1016/j.neulet.2013.12.030. Epub 2013 Dec 19. Neurosci Lett. 2014. PMID: 24361547 Free PMC article. Review.
-
Nucleotide signaling in astrogliosis.Neurosci Lett. 2014 Apr 17;565:14-22. doi: 10.1016/j.neulet.2013.09.056. Epub 2013 Oct 5. Neurosci Lett. 2014. PMID: 24103370 Review.
Cited by
-
Targeting the thrombin receptor modulates inflammation and astrogliosis to improve recovery after spinal cord injury.Neurobiol Dis. 2016 Sep;93:226-42. doi: 10.1016/j.nbd.2016.04.010. Epub 2016 May 1. Neurobiol Dis. 2016. PMID: 27145117 Free PMC article.
-
Role of the protease-activated receptor 1 in regulating the function of glial cells within central and peripheral nervous system.J Neural Transm (Vienna). 2019 Oct;126(10):1259-1271. doi: 10.1007/s00702-019-02075-z. Epub 2019 Sep 6. J Neural Transm (Vienna). 2019. PMID: 31493095 Review.
-
Differential expression of multiple kallikreins in a viral model of multiple sclerosis points to unique roles in the innate and adaptive immune response.Biol Chem. 2014 Sep;395(9):1063-73. doi: 10.1515/hsz-2014-0141. Biol Chem. 2014. PMID: 25153387 Free PMC article.
-
Toll-Like Receptor 4 (TLR4) and Triggering Receptor Expressed on Myeloid Cells-2 (TREM-2) Activation Balance Astrocyte Polarization into a Proinflammatory Phenotype.Mol Neurobiol. 2018 May;55(5):3875-3888. doi: 10.1007/s12035-017-0618-z. Epub 2017 May 25. Mol Neurobiol. 2018. PMID: 28547529
-
Astrocyte heterogeneity across the brain and spinal cord occurs developmentally, in adulthood and in response to demyelination.PLoS One. 2017 Jul 10;12(7):e0180697. doi: 10.1371/journal.pone.0180697. eCollection 2017. PLoS One. 2017. PMID: 28700615 Free PMC article.
References
-
- Abe K, Saito H. The p44/42 mitogen-activated protein kinase cascade is involved in the induction and maintenance of astrocyte stellation mediated by protein kinase C. Neurosci Res. 2000;36:251–257. - PubMed
-
- Adams MN, Ramachandran R, Yau MK, Suen JY, Fairlie DP, Hollenberg MD, Hooper JD. Structure, function and pathophysiology of protease activated receptors. Pharmacol Ther 2011 - PubMed
-
- Angelo PF, Lima AR, Alves FM, Blaber SI, Scarisbrick IA, Blaber M, Juliano L, Juliano MA. Substrate specificity of human kallikrein 6: salt and glycosaminoglycan activation effects. The Journal of biological chemistry. 2006;281:3116–3126. - PubMed
-
- Bernett MJ, Blaber SI, Scarisbrick IA, Dhanarajan P, Thompson SM, Blaber M. Crystal structure and biochemical characterization of human kallikrein 6 reveals that a trypsin-like kallikrein is expressed in the central nervous system. The Journal of biological chemistry. 2002;277:24562–24570. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
