Human heat shock protein 105/110 kDa (Hsp105/110) regulates biogenesis and quality control of misfolded cystic fibrosis transmembrane conductance regulator at multiple levels
- PMID: 22505710
- PMCID: PMC3365948
- DOI: 10.1074/jbc.M111.297580
Human heat shock protein 105/110 kDa (Hsp105/110) regulates biogenesis and quality control of misfolded cystic fibrosis transmembrane conductance regulator at multiple levels
Abstract
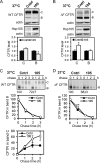
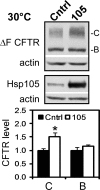
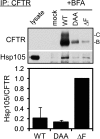
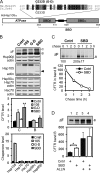
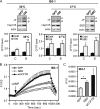
Heat shock protein 105/110-kDa (Hsp105/110), a member of the Hsp70 super family of molecular chaperones, serves as a nucleotide exchange factor for Hsc70, independently prevents the aggregation of misfolded proteins, and functionally relates to Hsp90. We investigated the roles of human Hsp105α, the constitutively expressed isoform, in the biogenesis and quality control of the cystic fibrosis transmembrane conductance regulator (CFTR). In the endoplasmic reticulum (ER), Hsp105 facilitates CFTR quality control at an early stage in its biosynthesis but promotes CFTR post-translational folding. Deletion of Phe-508 (ΔF508), the most prevalent mutation causing cystic fibrosis, interferes with de novo folding of CFTR, impairing its export from the ER and accelerating its clearance in the ER and post-Golgi compartments. We show that Hsp105 preferentially associates with and stabilizes ΔF508 CFTR at both levels. Introduction of the Hsp105 substrate binding domain potently increases the steady state level of ΔF508 CFTR by reducing its early-stage degradation. This in turn dramatically enhances ΔF508 CFTR cell surface functional expression in cystic fibrosis airway epithelial cells. Although other Hsc70 nucleotide exchange factors such as HspBP1 and BAG-2 inhibit CFTR post-translational degradation in the ER through cochaperone CHIP, Hsp105 has a primary role promoting CFTR quality control at an earlier stage. The Hsp105-mediated multilevel regulation of ΔF508 CFTR folding and quality control provides new opportunities to understand how chaperone machinery regulates the homeostasis and functional expression of misfolded proteins in the cell. Future studies in this direction will inform therapeutics development for cystic fibrosis and other protein misfolding diseases.
Figures


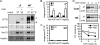

Similar articles
-
Matrine modulates HSC70 levels and rescues ΔF508-CFTR.J Cell Physiol. 2012 Sep;227(9):3317-23. doi: 10.1002/jcp.24028. J Cell Physiol. 2012. PMID: 22170045
-
Silencing of the Hsp70-specific nucleotide-exchange factor BAG3 corrects the F508del-CFTR variant by restoring autophagy.J Biol Chem. 2018 Aug 31;293(35):13682-13695. doi: 10.1074/jbc.RA118.002607. Epub 2018 Jul 9. J Biol Chem. 2018. PMID: 29986884 Free PMC article.
-
Control of cystic fibrosis transmembrane conductance regulator membrane trafficking: not just from the endoplasmic reticulum to the Golgi.FEBS J. 2013 Sep;280(18):4396-406. doi: 10.1111/febs.12392. Epub 2013 Jul 5. FEBS J. 2013. PMID: 23773658 Review.
-
AAV exploits subcellular stress associated with inflammation, endoplasmic reticulum expansion, and misfolded proteins in models of cystic fibrosis.PLoS Pathog. 2011 May;7(5):e1002053. doi: 10.1371/journal.ppat.1002053. Epub 2011 May 19. PLoS Pathog. 2011. PMID: 21625534 Free PMC article.
-
Cystic fibrosis transmembrane conductance regulator degradation: cross-talk between the ubiquitylation and SUMOylation pathways.FEBS J. 2013 Sep;280(18):4430-8. doi: 10.1111/febs.12415. Epub 2013 Jul 22. FEBS J. 2013. PMID: 23809253 Free PMC article. Review.
Cited by
-
Role of BAG5 in Protein Quality Control: Double-Edged Sword?Front Aging. 2022 Mar 3;3:844168. doi: 10.3389/fragi.2022.844168. eCollection 2022. Front Aging. 2022. PMID: 35821856 Free PMC article. Review.
-
Transcriptomic and Proteostasis Networks of CFTR and the Development of Small Molecule Modulators for the Treatment of Cystic Fibrosis Lung Disease.Genes (Basel). 2020 May 13;11(5):546. doi: 10.3390/genes11050546. Genes (Basel). 2020. PMID: 32414011 Free PMC article. Review.
-
Three epilepsy-associated GABRG2 missense mutations at the γ+/β- interface disrupt GABAA receptor assembly and trafficking by similar mechanisms but to different extents.Neurobiol Dis. 2014 Aug;68:167-79. doi: 10.1016/j.nbd.2014.04.015. Epub 2014 May 4. Neurobiol Dis. 2014. PMID: 24798517 Free PMC article.
-
Metazoan Hsp70-based protein disaggregases: emergence and mechanisms.Front Mol Biosci. 2015 Oct 9;2:57. doi: 10.3389/fmolb.2015.00057. eCollection 2015. Front Mol Biosci. 2015. PMID: 26501065 Free PMC article. Review.
-
HDAC inhibitors rescue multiple disease-causing CFTR variants.Hum Mol Genet. 2019 Jun 15;28(12):1982-2000. doi: 10.1093/hmg/ddz026. Hum Mol Genet. 2019. PMID: 30753450 Free PMC article.
References
-
- Lee-Yoon D., Easton D., Murawski M., Burd R., Subjeck J. R. (1995) Identification of a major subfamily of large hsp70-like proteins through the cloning of the mammalian 110-kDa heat shock protein. J. Biol. Chem. 270, 15725–15733 - PubMed
-
- Oh H. J., Chen X., Subjeck J. R. (1997) Hsp110 protects heat-denatured proteins and confers cellular thermoresistance. J. Biol. Chem. 272, 31636–31640 - PubMed
-
- Oh H. J., Easton D., Murawski M., Kaneko Y., Subjeck J. R. (1999) The chaperoning activity of hsp110. Identification of functional domains by use of targeted deletions. J. Biol. Chem. 274, 15712–15718 - PubMed
-
- Hatayama T., Yasuda K., Yasuda K. (1998) Association of HSP105 with HSC70 in high molecular mass complexes in mouse FM3A cells. Biochem. Biophys. Res. Commun. 248, 395–401 - PubMed
-
- Yamagishi N., Ishihara K., Hatayama T. (2004) Hsp105α suppresses Hsc70 chaperone activity by inhibiting Hsc70 ATPase activity. J. Biol. Chem. 279, 41727–41733 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

