Biologically relevant mechanism for catalytic superoxide removal by simple manganese compounds
- PMID: 22505740
- PMCID: PMC3344976
- DOI: 10.1073/pnas.1203051109
Biologically relevant mechanism for catalytic superoxide removal by simple manganese compounds
Abstract
Nonenzymatic manganese was first shown to provide protection against superoxide toxicity in vivo in 1981, but the chemical mechanism responsible for this protection subsequently became controversial due to conflicting reports concerning the ability of Mn to catalyze superoxide disproportionation in vitro. In a recent communication, we reported that low concentrations of a simple Mn phosphate salt under physiologically relevant conditions will indeed catalyze superoxide disproportionation in vitro. We report now that two of the four Mn complexes that are expected to be most abundant in vivo, Mn phosphate and Mn carbonate, can catalyze superoxide disproportionation at physiologically relevant concentrations and pH, whereas Mn pyrophosphate and citrate complexes cannot. Additionally, the chemical mechanisms of these reactions have been studied in detail, and the rates of reactions of the catalytic removal of superoxide by Mn phosphate and carbonate have been modeled. Physiologically relevant concentrations of these compounds were found to be sufficient to mimic an effective concentration of enzymatic superoxide dismutase found in vivo. This mechanism provides a likely explanation as to how Mn combats superoxide stress in cellular systems.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
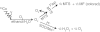
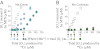



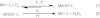
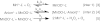




References
-
- Daly MJ, et al. Accumulation of Mn(II) in Deinococcus radiodurans facilitates gamma-radiation resistance. Science. 2004;306:1025–1028. - PubMed
-
- Chang EC, Kosman DJ. Intracellular Mn (II)-associated superoxide scavenging activity protects Cu,Zn superoxide dismutase-deficient Saccharomyces cerevisiae against dioxygen stress. J Biol Chem. 1989;264:12172–12178. - PubMed
-
- Tseng HJ, Srikhanta Y, McEwan AG, Jennings MP. Accumulation of manganese in Neisseria gonorrhoeae correlates with resistance to oxidative killing by superoxide anion and is independent of superoxide dismutase activity. Mol Microbiol. 2001;40:1175–1186. - PubMed
-
- Al-Maghrebi M, Fridovich I, Benov L. Manganese supplementation relieves the phenotypic deficits seen in superoxide-dismutase-null Escherichia coli. Arch Biochem Biophys. 2002;402:104–109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

