Integrins and their extracellular matrix ligands in lymphangiogenesis and lymph node metastasis
- PMID: 22505936
- PMCID: PMC3296286
- DOI: 10.1155/2012/853703
Integrins and their extracellular matrix ligands in lymphangiogenesis and lymph node metastasis
Abstract
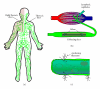
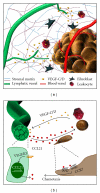
In the 1970s, the late Judah Folkman postulated that tumors grow proportionately to their blood supply and that tumor angiogenesis removed this limitation promoting growth and metastasis. Work over the past 40 years, varying from molecular examination to clinical trials, verified this hypothesis and identified a host of therapeutic targets to limit tumor angiogenesis, including the integrin family of extracellular matrix receptors. However, the propensity for some tumors to spread through lymphatics suggests that lymphangiogenesis plays a similarly important role. Lymphangiogenesis inhibitors reduce lymph node metastasis, the leading indicator of poor prognosis, whereas inducing lymphangiogenesis promotes lymph node metastasis even in cancers not prone to lymphatic dissemination. Recent works highlight a role for integrins in lymphangiogenesis and suggest that integrin inhibitors may serve as therapeutic targets to limit lymphangiogenesis and lymph node metastasis. This review discusses the current literature on integrin-matrix interactions in lymphatic vessel development and lymphangiogenesis and highlights our current knowledge on how specific integrins regulate tumor lymphangiogenesis.
Figures

Similar articles
-
Roles of integrins in tumor angiogenesis and lymphangiogenesis.Lymphat Res Biol. 2008;6(3-4):155-63. doi: 10.1089/lrb.2008.1011. Lymphat Res Biol. 2008. PMID: 19093788 Free PMC article. Review.
-
Integrins in tumor angiogenesis and lymphangiogenesis.Methods Mol Biol. 2012;757:471-86. doi: 10.1007/978-1-61779-166-6_27. Methods Mol Biol. 2012. PMID: 21909928 Free PMC article.
-
Tumor-secreted vascular endothelial growth factor-C is necessary for prostate cancer lymphangiogenesis, but lymphangiogenesis is unnecessary for lymph node metastasis.Cancer Res. 2005 Nov 1;65(21):9789-98. doi: 10.1158/0008-5472.CAN-05-0901. Cancer Res. 2005. PMID: 16267000
-
Lymphatic vessel density is not associated with lymph node metastasis in non-small cell lung carcinoma.Arch Pathol Lab Med. 2008 Dec;132(12):1882-8. doi: 10.5858/132.12.1882. Arch Pathol Lab Med. 2008. PMID: 19061284
-
Lymphangiogenesis, inflammation and metastasis.Anticancer Res. 2005 Nov-Dec;25(6C):4503-11. Anticancer Res. 2005. PMID: 16334134 Review.
Cited by
-
Mechanisms of Tumor-Induced Lymphovascular Niche Formation in Draining Lymph Nodes.Cell Rep. 2018 Dec 26;25(13):3554-3563.e4. doi: 10.1016/j.celrep.2018.12.002. Cell Rep. 2018. PMID: 30590031 Free PMC article.
-
A novel 3D culture model recapitulates primary FL B-cell features and promotes their survival.Blood Adv. 2021 Dec 14;5(23):5372-5386. doi: 10.1182/bloodadvances.2020003949. Blood Adv. 2021. PMID: 34555842 Free PMC article.
-
Characterization of Photo-Crosslinked Methacrylated Type I Collagen as a Platform to Investigate the Lymphatic Endothelial Cell Response.Lymphatics. 2024 Sep;2(3):177-194. doi: 10.3390/lymphatics2030015. Epub 2024 Sep 19. Lymphatics. 2024. PMID: 39664172 Free PMC article.
-
Disrupted Endothelial Cell Layer and Exposed Extracellular Matrix Proteins Promote Capture of Late Outgrowth Endothelial Progenitor Cells.Stem Cells Int. 2016;2016:1406304. doi: 10.1155/2016/1406304. Epub 2016 Jun 16. Stem Cells Int. 2016. PMID: 27413378 Free PMC article.
-
Evaluation of MR elastography for prediction of lymph node metastasis in prostate cancer.Abdom Radiol (NY). 2021 Jul;46(7):3387-3400. doi: 10.1007/s00261-021-02982-4. Epub 2021 Mar 2. Abdom Radiol (NY). 2021. PMID: 33651125 Free PMC article.
References
-
- Földi M, Strössenreuther R. Foundations of Manual Lymph Drainage. 3rd edition. New York, NY, USA: Elsevier; 2004.
-
- Komarova Y, Malik AB. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annual Review of Physiology. 2009;72:463–493. - PubMed
-
- Weller RO, Galea I, Carare RO, Minagar A. Pathophysiology of the lymphatic drainage of the central nervous system: implications for pathogenesis and therapy of multiple sclerosis. Pathophysiology. 2010;17(4):295–306. - PubMed
-
- Oliver G. Lymphatic vasculature development. Nature Reviews Immunology. 2004;4(1):35–45. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

