Dynamics of Lymphocyte Populations during Trypanosoma cruzi Infection: From Thymocyte Depletion to Differential Cell Expansion/Contraction in Peripheral Lymphoid Organs
- PMID: 22505943
- PMCID: PMC3306984
- DOI: 10.1155/2012/747185
Dynamics of Lymphocyte Populations during Trypanosoma cruzi Infection: From Thymocyte Depletion to Differential Cell Expansion/Contraction in Peripheral Lymphoid Organs
Abstract
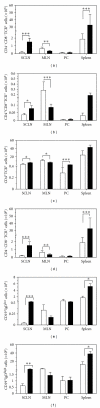
The comprehension of the immune responses in infectious diseases is crucial for developing novel therapeutic strategies. Here, we review current findings on the dynamics of lymphocyte subpopulations following experimental acute infection by Trypanosoma cruzi, the causative agent of Chagas disease. In the thymus, although the negative selection process of the T-cell repertoire remains operational, there is a massive thymocyte depletion and abnormal release of immature CD4(+)CD8(+) cells to peripheral lymphoid organs, where they acquire an activated phenotype similar to activated effector or memory T cells. These cells apparently bypassed the negative selection process, and some of them are potentially autoimmune. In infected animals, an atrophy of mesenteric lymph nodes is also observed, in contrast with the lymphocyte expansion in spleen and subcutaneous lymph nodes, illustrating a complex and organ specific dynamics of lymphocyte subpopulations. Accordingly, T- and B-cell activation is seen in subcutaneous lymph nodes and spleen, but not in mesenteric lymph nodes. Lastly, although the function of peripheral CD4(+)CD8(+) T-cell population remains to be defined in vivo, their presence may contribute to the immunopathological events found in both murine and human Chagas disease.
Figures

Similar articles
-
Experimental Trypanosoma cruzi infection alters the shaping of the central and peripheral T-cell repertoire.Microbes Infect. 2003 Aug;5(10):825-32. doi: 10.1016/s1286-4579(03)00156-4. Microbes Infect. 2003. PMID: 12919850
-
Differential regional immune response in Chagas disease.PLoS Negl Trop Dis. 2009 Jul 7;3(7):e417. doi: 10.1371/journal.pntd.0000417. PLoS Negl Trop Dis. 2009. PMID: 19582140 Free PMC article. Review.
-
Chagasic thymic atrophy does not affect negative selection but results in the export of activated CD4+CD8+ T cells in severe forms of human disease.PLoS Negl Trop Dis. 2011 Aug;5(8):e1268. doi: 10.1371/journal.pntd.0001268. Epub 2011 Aug 16. PLoS Negl Trop Dis. 2011. PMID: 21858238 Free PMC article.
-
TNF-α is involved in the abnormal thymocyte migration during experimental Trypanosoma cruzi infection and favors the export of immature cells.PLoS One. 2012;7(3):e34360. doi: 10.1371/journal.pone.0034360. Epub 2012 Mar 26. PLoS One. 2012. PMID: 22461911 Free PMC article.
-
Molecular mechanisms governing thymocyte migration: combined role of chemokines and extracellular matrix.J Leukoc Biol. 2004 Jun;75(6):951-61. doi: 10.1189/jlb.1003455. Epub 2004 Mar 12. J Leukoc Biol. 2004. PMID: 15020651 Review.
Cited by
-
IFNγ and iNOS-Mediated Alterations in the Bone Marrow and Thymus and Its Impact on Mycobacterium avium-Induced Thymic Atrophy.Front Immunol. 2021 Dec 20;12:696415. doi: 10.3389/fimmu.2021.696415. eCollection 2021. Front Immunol. 2021. PMID: 34987496 Free PMC article.
-
Decreased level of antibodies and cardiac involvement in patients with chronic Chagas heart disease vaccinated with BCG.Med Microbiol Immunol. 2014 Apr;203(2):133-9. doi: 10.1007/s00430-013-0326-x. Epub 2013 Dec 29. Med Microbiol Immunol. 2014. PMID: 24374613
-
Altered bone marrow lymphopoiesis and interleukin-6-dependent inhibition of thymocyte differentiation contribute to thymic atrophy during Trypanosoma cruzi infection.Oncotarget. 2017 Mar 14;8(11):17551-17561. doi: 10.18632/oncotarget.14886. Oncotarget. 2017. PMID: 28147332 Free PMC article.
-
Detection of amastigotes and histopathological alterations in the thymus of Leishmania infantum-infected dogs.Immun Inflamm Dis. 2020 Jun;8(2):127-139. doi: 10.1002/iid3.285. Epub 2020 Mar 24. Immun Inflamm Dis. 2020. PMID: 32207879 Free PMC article.
-
A synthetic peptide from Trypanosoma cruzi mucin-like associated surface protein as candidate for a vaccine against Chagas disease.Vaccine. 2014 Jun 12;32(28):3525-32. doi: 10.1016/j.vaccine.2014.04.026. Epub 2014 Apr 30. Vaccine. 2014. PMID: 24793944 Free PMC article.
References
-
- Parker ER, Sethi A. Chagas disease: coming to a place near you. Dermatologic Clinics. 2011;29(1):53–62. - PubMed
-
- Coura JR, Dias JCP. Epidemiology, control and surveillance of Chagas disease—100 years after its discovery. Memorias do Instituto Oswaldo Cruz. 2009;104(supplement 1):31–40. - PubMed
-
- Punukollu G, Gowda RM, Khan IA, Navarro VS, Vasavada BC. Clinical aspects of the Chagas’ heart disease. International Journal of Cardiology. 2007;115(3):279–283. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

