Microfluidic sorting of microtissues
- PMID: 22505992
- PMCID: PMC3324260
- DOI: 10.1063/1.3692765
Microfluidic sorting of microtissues
Abstract
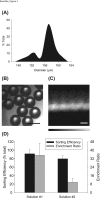
Increasingly, invitro culture of adherent cell types utilizes three-dimensional (3D) scaffolds or aggregate culture strategies to mimic tissue-like, microenvironmental conditions. In parallel, new flow cytometry-based technologies are emerging to accurately analyze the composition and function of these microtissues (i.e., large particles) in a non-invasive and high-throughput way. Lacking, however, is an accessible platform that can be used to effectively sort or purify large particles based on analysis parameters. Here we describe a microfluidic-based, electromechanical approach to sort large particles. Specifically, sheath-less asymmetric curving channels were employed to separate and hydrodynamically focus particles to be analyzed and subsequently sorted. This design was developed and characterized based on wall shear stress, tortuosity of the flow path, vorticity of the fluid in the channel, sorting efficiency and enrichment ratio. The large particle sorting device was capable of purifying fluorescently labelled embryoid bodies (EBs) from unlabelled EBs with an efficiency of 87.3% ± 13.5%, and enrichment ratio of 12.2 ± 8.4 (n = 8), while preserving cell viability, differentiation potential, and long-term function.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
