Heme oxygenase-1 induction and organic nitrate therapy: beneficial effects on endothelial dysfunction, nitrate tolerance, and vascular oxidative stress
- PMID: 22506100
- PMCID: PMC3312327
- DOI: 10.1155/2012/842632
Heme oxygenase-1 induction and organic nitrate therapy: beneficial effects on endothelial dysfunction, nitrate tolerance, and vascular oxidative stress
Abstract
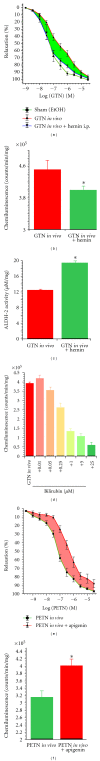
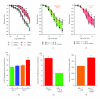
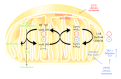
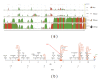
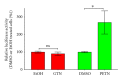
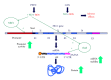
Organic nitrates are a group of very effective anti-ischemic drugs. They are used for the treatment of patients with stable angina, acute myocardial infarction, and chronic congestive heart failure. A major therapeutic limitation inherent to organic nitrates is the development of tolerance, which occurs during chronic treatment with these agents, and this phenomenon is largely based on induction of oxidative stress with subsequent endothelial dysfunction. We therefore speculated that induction of heme oxygenase-1 (HO-1) could be an efficient strategy to overcome nitrate tolerance and the associated side effects. Indeed, we found that hemin cotreatment prevented the development of nitrate tolerance and vascular oxidative stress in response to chronic nitroglycerin therapy. Vice versa, pentaerithrityl tetranitrate (PETN), a nitrate that was previously reported to be devoid of adverse side effects, displayed tolerance and oxidative stress when the HO-1 pathway was blocked pharmacologically or genetically by using HO-1(+/-) mice. Recently, we identified activation of Nrf2 and HuR as a principle mechanism of HO-1 induction by PETN. With the present paper, we present and discuss our recent and previous findings on the role of HO-1 for the prevention of nitroglycerin-induced nitrate tolerance and for the beneficial effects of PETN therapy.
Figures



References
-
- Munzel T, Daiber A, Mulsch A. Explaining the phenomenon of nitrate tolerance. Circulation Research. 2005;97(7):618–628. - PubMed
-
- Munzel T, Daiber A, Gori T. Nitrate therapy: new aspects concerning molecular action and tolerance. Circulation. 2011;123(19):2132–2144. - PubMed
-
- Daiber A, Munzel T, Gori T. Organic nitrates and nitrate tolerance—state of the art and future developments. Advances in Pharmacology. 2010;60:177–227. - PubMed
-
- Daiber A, Oelze M, Wenzel P, et al. Nitrate tolerance as a model of vascular dysfunction: roles for mitochondrial aldehyde dehydrogenase and mitochondrial oxidative stress. Pharmacological Reports. 2009;61(1):33–48. - PubMed
-
- Daiber A, Wenzel P, Oelze M, Munzel T. New insights into bioactivation of organic nitrates, nitrate tolerance and cross-tolerance. Clinical Research in Cardiology. 2008;97(1):12–20. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

