Synthesis and biological evaluation of new potent and selective HCV NS5A inhibitors
- PMID: 22507961
- PMCID: PMC7732024
- DOI: 10.1016/j.bmcl.2012.03.089
Synthesis and biological evaluation of new potent and selective HCV NS5A inhibitors
Abstract
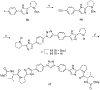
NS5A inhibitors are a new class of direct-acting antiviral agents which display very potent anti-HCV activity in vitro and in humans. Rationally designed modifications to the central biphenyl linkage of a known NS5A series led to selection of several compounds that were synthesized and evaluated in a HCV genotype 1b replicon. The straight triphenyl linked compound 11a showed similar anti-HCV activity to the clinical compound BMS-790052 and a superior cytotoxicity profile in three different cell lines, with an EC(50) value of 26 pM and a therapeutic index of over four million in an HCV replicon assay. This triphenyl analog warrants further preclinical evaluation as an anti-HCV agent.
Copyright © 2012. Published by Elsevier Ltd.
Figures




Similar articles
-
Synthesis and evaluation of novel HCV replication inhibitors.Mol Divers. 2017 May;21(2):475-481. doi: 10.1007/s11030-017-9733-z. Epub 2017 Mar 14. Mol Divers. 2017. PMID: 28293834
-
Synthesis and evaluation of non-dimeric HCV NS5A inhibitors.Bioorg Med Chem Lett. 2013 Apr 1;23(7):2031-4. doi: 10.1016/j.bmcl.2013.02.023. Epub 2013 Feb 13. Bioorg Med Chem Lett. 2013. PMID: 23466233 Free PMC article.
-
Discovery of fused tricyclic core containing HCV NS5A inhibitors with pan-genotype activity.Bioorg Med Chem Lett. 2016 Jul 1;26(13):3158-3162. doi: 10.1016/j.bmcl.2016.04.084. Epub 2016 Apr 30. Bioorg Med Chem Lett. 2016. PMID: 27180013
-
Antiviral activity and resistance of HCV NS5A replication complex inhibitors.Curr Opin Virol. 2013 Oct;3(5):514-20. doi: 10.1016/j.coviro.2013.06.014. Epub 2013 Jul 27. Curr Opin Virol. 2013. PMID: 23896281 Review.
-
[Research progress of HCV NS5A complex inhibitors].Yao Xue Xue Bao. 2016 Sep;51(9):1378-87. Yao Xue Xue Bao. 2016. PMID: 29924511 Review. Chinese.
Cited by
-
Potent Hepatitis C Virus NS5A Inhibitors Containing a Benzidine Core.ACS Med Chem Lett. 2013 Dec 4;5(3):255-8. doi: 10.1021/ml4003293. eCollection 2014 Mar 13. ACS Med Chem Lett. 2013. PMID: 24900814 Free PMC article.
-
Design, synthesis and evaluation of novel anti-HCV molecules that deliver intracellularly three highly potent NS5A inhibitors.Bioorg Med Chem Lett. 2015 Sep 1;25(17):3711-5. doi: 10.1016/j.bmcl.2015.06.031. Epub 2015 Jun 15. Bioorg Med Chem Lett. 2015. PMID: 26099532 Free PMC article.
-
Synthesis and evaluation of novel HCV replication inhibitors.Mol Divers. 2017 May;21(2):475-481. doi: 10.1007/s11030-017-9733-z. Epub 2017 Mar 14. Mol Divers. 2017. PMID: 28293834
-
Approaches to hepatitis C treatment and cure using NS5A inhibitors.Infect Drug Resist. 2014 Mar 5;7:41-56. doi: 10.2147/IDR.S36247. eCollection 2014. Infect Drug Resist. 2014. PMID: 24623983 Free PMC article. Review.
-
Asymmetric binding to NS5A by daclatasvir (BMS-790052) and analogs suggests two novel modes of HCV inhibition.J Med Chem. 2014 Dec 11;57(23):10031-43. doi: 10.1021/jm501291c. Epub 2014 Nov 3. J Med Chem. 2014. PMID: 25365735 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

