Mipomersen, an apolipoprotein B synthesis inhibitor, lowers low-density lipoprotein cholesterol in high-risk statin-intolerant patients: a randomized, double-blind, placebo-controlled trial
- PMID: 22507979
- PMCID: PMC3751967
- DOI: 10.1093/eurheartj/ehs023
Mipomersen, an apolipoprotein B synthesis inhibitor, lowers low-density lipoprotein cholesterol in high-risk statin-intolerant patients: a randomized, double-blind, placebo-controlled trial
Abstract
Aims: A randomized, double-blind, placebo-controlled study was conducted to investigate the safety and efficacy of mipomersen, an apolipoprotein B-100 (apoB) synthesis inhibitor, in patients who are statin intolerant and at high risk for cardiovascular disease (CVD).
Methods and results: Thirty-three subjects, not receiving statin therapy because of statin intolerance, received a weekly subcutaneous dose of 200 mg mipomersen or placebo (2:1 randomization) for 26 weeks. The primary endpoint was per cent change in LDL cholesterol (LDL-c) from the baseline to Week 28. The other efficacy endpoints were per cent change in apoB and lipoprotein a [Lp(a)]. Safety was determined using the incidence of treatment-emergent adverse events (AEs) and clinical laboratory evaluations. After 26 weeks of mipomersen administration, LDL-c was reduced by 47 ± 18% (P < 0.001 vs. placebo). apoB and Lp(a) were also significantly reduced by 46 and 27%, respectively (P < 0.001 vs. placebo). Four mipomersen (19%) and two placebo subjects (17%) discontinued dosing prematurely due to AEs. Persistent liver transaminase increases ≥ 3× the upper limit of normal were observed in seven (33%) subjects assigned to mipomersen. In selected subjects, liver fat content was assessed, during and after treatment, using magnetic resonance spectroscopy. Liver fat content in these patients ranged from 0.8 to 47.3%. Liver needle biopsy was performed in two of these subjects, confirming hepatic steatosis with minimal inflammation or fibrosis.
Conclusion: The present data suggest that mipomersen is a potential therapeutic option in statin-intolerant patients at high risk for CVD. The long-term follow-up of liver safety is required.
Clinical trial registration: ClinicalTrials.gov identifier: NCT00707746.
Figures
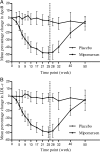


Comment in
-
A new approach to managing the 'statin-intolerant' patient?Eur Heart J. 2012 May;33(9):1040-3. doi: 10.1093/eurheartj/ehs062. Epub 2012 Apr 16. Eur Heart J. 2012. PMID: 22507980 No abstract available.
References
-
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment III) Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment III) final report. Circulation. 2002;106:3143–3421. - PubMed
-
- Bruckert E, Hayem G, Dejager S, Yau C, Begaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther. 2005;19:403–414. - PubMed
-
- Harper CR, Jacobson TA. The broad spectrum of statin myopathy: from myalgia to rhabdomyolysis. Curr Opin Lipidol. 2007;18:401–408. - PubMed
-
- McKenney JM, Davidson MH, Jacobson TA, Guyton JR. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol. 2006;97:89C–94C. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

