Cep57, a NEDD1-binding pericentriolar material component, is essential for spindle pole integrity
- PMID: 22508265
- PMCID: PMC3434346
- DOI: 10.1038/cr.2012.61
Cep57, a NEDD1-binding pericentriolar material component, is essential for spindle pole integrity
Abstract
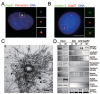
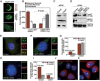
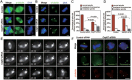
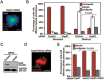
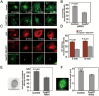
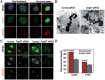
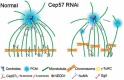
Formation of a bipolar spindle is indispensable for faithful chromosome segregation and cell division. Spindle integrity is largely dependent on the centrosome and the microtubule network. Centrosome protein Cep57 can bundle microtubules in mammalian cells. Its related protein (Cep57R) in Xenopus was characterized as a stabilization factor for microtubule-kinetochore attachment. Here we show that Cep57 is a pericentriolar material (PCM) component. Its interaction with NEDD1 is necessary for the centrosome localization of Cep57. Depletion of Cep57 leads to unaligned chromosomes and a multipolar spindle, which is induced by PCM fragmentation. In the absence of Cep57, centrosome microtubule array assembly activity is weakened, and the spindle length and microtubule density decrease. As a spindle microtubule-binding protein, Cep57 is also responsible for the proper organization of the spindle microtubule and localization of spindle pole focusing proteins. Collectively, these results suggest that Cep57, as a NEDD1-binding centrosome component, could function as a spindle pole- and microtubule-stabilizing factor for establishing robust spindle architecture.
Figures

Similar articles
-
The Cep57-pericentrin module organizes PCM expansion and centriole engagement.Nat Commun. 2019 Feb 25;10(1):931. doi: 10.1038/s41467-019-08862-2. Nat Commun. 2019. PMID: 30804344 Free PMC article.
-
Cep57 protein is required for cytokinesis by facilitating central spindle microtubule organization.J Biol Chem. 2013 May 17;288(20):14384-14390. doi: 10.1074/jbc.M112.441501. Epub 2013 Apr 8. J Biol Chem. 2013. PMID: 23569207 Free PMC article.
-
CENP-W plays a role in maintaining bipolar spindle structure.PLoS One. 2014 Oct 15;9(10):e106464. doi: 10.1371/journal.pone.0106464. eCollection 2014. PLoS One. 2014. PMID: 25329824 Free PMC article.
-
Microtubule attachment and spindle assembly checkpoint signalling at the kinetochore.Nat Rev Mol Cell Biol. 2013 Jan;14(1):25-37. doi: 10.1038/nrm3494. Nat Rev Mol Cell Biol. 2013. PMID: 23258294 Free PMC article. Review.
-
Mechanisms of Mitotic Spindle Assembly.Annu Rev Biochem. 2016 Jun 2;85:659-83. doi: 10.1146/annurev-biochem-060815-014528. Epub 2016 Apr 21. Annu Rev Biochem. 2016. PMID: 27145846 Free PMC article. Review.
Cited by
-
α-/γ-Taxilin are required for centriolar subdistal appendage assembly and microtubule organization.Elife. 2022 Feb 4;11:e73252. doi: 10.7554/eLife.73252. Elife. 2022. PMID: 35119360 Free PMC article.
-
The Cep57-pericentrin module organizes PCM expansion and centriole engagement.Nat Commun. 2019 Feb 25;10(1):931. doi: 10.1038/s41467-019-08862-2. Nat Commun. 2019. PMID: 30804344 Free PMC article.
-
Prognostic Significance and Functional Role of CEP57 in Prostate Cancer.Transl Oncol. 2015 Dec;8(6):487-96. doi: 10.1016/j.tranon.2015.11.004. Transl Oncol. 2015. PMID: 26692530 Free PMC article.
-
Proximity Interactions among Basal Body Components in Trypanosoma brucei Identify Novel Regulators of Basal Body Biogenesis and Inheritance.mBio. 2017 Jan 3;8(1):e02120-16. doi: 10.1128/mBio.02120-16. mBio. 2017. PMID: 28049148 Free PMC article.
-
A self-assembled cylindrical platform for Plk4-induced centriole biogenesis.Open Biol. 2020 Aug;10(8):200102. doi: 10.1098/rsob.200102. Epub 2020 Aug 19. Open Biol. 2020. PMID: 32810424 Free PMC article. Review.
References
-
- Blagden SP, Glover DM. Polar expeditions – provisioning the centrosome for mitosis. Nat Cell Biol. 2003;5:505–511. - PubMed
-
- Zheng Y, Wong ML, Alberts B, Mitchison T. Nucleation of microtubule assembly by a gamma-tubulin-containing ring complex. Nature. 1995;378:578–583. - PubMed
-
- Palazzo RE, Vogel JM, Schnackenberg BJ, Hull DR, Wu X. Centrosome maturation. Curr Top Dev Biol. 2000;49:449–470. - PubMed
-
- Oshimori N, Ohsugi M, Yamamoto T. The Plk1 target Kizuna stabilizes mitotic centrosomes to ensure spindle bipolarity. Nat Cell Biol. 2006;8:1095–1101. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

