Premotor Parkinson's disease: concepts and definitions
- PMID: 22508279
- PMCID: PMC3335740
- DOI: 10.1002/mds.24954
Premotor Parkinson's disease: concepts and definitions
Abstract
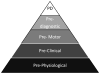
Parkinson's disease (PD) has a prodromal phase during which nonmotor clinical features as well as physiological abnormalities may be present. These premotor markers could be used to screen for PD before motor abnormalities are present. The technology to identify PD before it reaches symptomatic Braak Stage 3 (substantia nigra compacta [SNc] involvement) already exists. The current challenge is to define the appropriate scope of use of predictive testing for PD. Imaging technologies such as dopamine transporter imaging currently offer the highest degree of accuracy for identifying premotor PD, but they are expensive as screening tools, and abnormalities on these studies would only be evident at Braak Stage 3 or higher. Efficiency is greatly enhanced by combining imaging with a prescreening test such as olfactory testing. This 2-step process has the potential to greatly reduce costs while retaining diagnostic accuracy. Alternatively, or in concert with this approach, evaluating high-risk populations (eg, patients with rapid eye movement behavior disorder or LRRK2 mutations) would enrich the sample for cases with underlying PD. Ultimately, the role of preclinical detection of PD will be determined by the ability of emerging therapies to influence clinical outcomes. As such, implementation of large-scale screening strategies awaits the arrival of clearly safe and effective therapies that address the underlying pathogenesis of PD. Future research will establish more definitive biomarkers capable of revealing the presence of disease in advance of SNc involvement with the promise of the potential for introducing disease-modifying therapy even before the development of evidence of dopamine deficiency.
Copyright © 2012 Movement Disorder Society.
Figures

Comment in
-
Prodromal diagnosis of Parkinson's disease.Mov Disord. 2012 Sep 15;27(11):1467. doi: 10.1002/mds.25151. Epub 2012 Aug 23. Mov Disord. 2012. PMID: 22926872 No abstract available.
References
-
- Stern MB. The preclinical detection of Parkinson’s disease: ready for prime time? Annals of Neurology. 2004;56:169–171. - PubMed
-
- Siderowf A, Stern MB. Preclinical diagnosis of Parkinson’s disease: are we there yet? Current Neurology & Neuroscience Reports. 2006;6(4):295–301. - PubMed
-
- Louis ED, Bennett DA. Mild parkinsonian signs: An overview of an emerging concept. Movement Disorders. 2007;22(12):1681–1688. - PubMed
-
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, et al. Current concepts in mild cognitive impairment. Archives of Neurology. 2001;58(12):1985–1992. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

