Evaluation of arylimidamides DB1955 and DB1960 as candidates against visceral leishmaniasis and Chagas' disease: in vivo efficacy, acute toxicity, pharmacokinetics, and toxicology studies
- PMID: 22508306
- PMCID: PMC3393454
- DOI: 10.1128/AAC.06404-11
Evaluation of arylimidamides DB1955 and DB1960 as candidates against visceral leishmaniasis and Chagas' disease: in vivo efficacy, acute toxicity, pharmacokinetics, and toxicology studies
Abstract
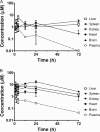
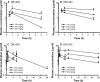
Arylimidamides (AIAs) have shown outstanding in vitro potency against intracellular kinetoplastid parasites, and the AIA 2,5-bis[2-(2-propoxy)-4-(2-pyridylimino)aminophenyl]furan dihydrochloride (DB766) displayed good in vivo efficacy in rodent models of visceral leishmaniasis (VL) and Chagas' disease. In an attempt to further increase the solubility and in vivo antikinetoplastid potential of DB766, the mesylate salt of this compound and that of the closely related AIA 2,5-bis[2-(2-cyclopentyloxy)-4-(2-pyridylimino)aminophenyl]furan hydrochloride (DB1852) were prepared. These two mesylate salts, designated DB1960 and DB1955, respectively, exhibited dose-dependent activity in the murine model of VL, with DB1960 inhibiting liver parasitemia by 51% at an oral dose of 100 mg/kg/day × 5 and DB1955 reducing liver parasitemia by 57% when given by the same dosing regimen. In a murine Trypanosoma cruzi infection model, DB1960 decreased the peak parasitemia levels that occurred at 8 days postinfection by 46% when given orally at 100 mg/kg/day × 5, while DB1955 had no effect on peak parasitemia levels when administered by the same dosing regimen. Distribution studies revealed that these compounds accumulated to micromolar levels in the liver, spleen, and kidneys but to a lesser extent in the heart, brain, and plasma. A 5-day repeat-dose toxicology study with DB1960 and DB1955 was also conducted with female BALB/c mice, with the compounds administered orally at 100, 200, and 500 mg/kg/day. In the high-dose groups, DB1960 caused changes in serum chemistry, with statistically significant increases in serum blood urea nitrogen, lactate dehydrogenase, aspartate aminotransferase, and alanine aminotransferase levels, and a 21% decrease in body weight was observed in this group. These changes were consistent with microscopic findings in the livers and kidneys of the treated animals. The incidences of observed clinical signs (hunched posture, tachypnea, tremors, and ruffled fur) were more frequent in DB1960-treated groups than in those treated with DB1955. However, histopathological examination of tissue samples indicated that both compounds had adverse effects at all dose levels.
Figures
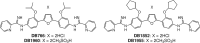


References
-
- Akay S. 2009. Diagnosis and inhibition tools in medicinal chemistry. Ph.D. dissertation Georgia State University, Atlanta, GA
-
- Berman J. 2005. Miltefosine to treat leishmaniasis. Expert Opin. Pharmacother. 6:1381–1388 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

