Activity of human immunodeficiency virus type 1 protease inhibitors against the initial autocleavage in Gag-Pol polyprotein processing
- PMID: 22508308
- PMCID: PMC3393404
- DOI: 10.1128/AAC.00055-12
Activity of human immunodeficiency virus type 1 protease inhibitors against the initial autocleavage in Gag-Pol polyprotein processing
Abstract
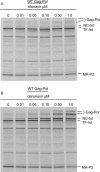
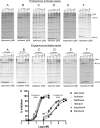
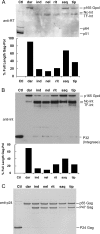
Inhibitors of HIV protease have proven to be important drugs in combination anti-HIV therapy. These inhibitors were designed to target mature protease and prevent viral particle maturation by blocking Gag and Gag-Pol processing by mature protease. Currently there are few data assessing the ability of these protease inhibitors to block the initial step in autoproteolytic processing of Gag-Pol. This unique step involves the dimerization of two Gag-Pol polyproteins and autocleavage of the Gag-Pol polyprotein by the embedded dimeric protease. We developed a plasmid encoding a modified form of Gag-Pol that can undergo autoprocessing only at the initial cleavage site between p2 and nucleocapsid. Using an in vitro transcription/translation system, we assessed the ability of six different approved protease inhibitors (darunavir, indinavir, nelfinavir, ritonavir, saquinavir, and tipranavir) to block this initial autocleavage step. Of these inhibitors, darunavir and saquinavir were the most effective. Darunavir and saquinavir were also the most effective at blocking the initial autoprocessing of full-length Gag-Pol in HIV-1-infected T cells. Thus, we have identified at least two HIV-1 protease inhibitors that have activity against the primary autocatalytic step of the embedded HIV-1 protease in Gag-Pol at concentrations that may be attained in HIV-1-infected patients. Due to unique aspects of the initial processing step, it may be possible to develop inhibitors with greater potency against this step, thus halting viral maturation at the earliest stages. The transcription/translation assay could be used to develop more potent inhibitors of this essential first step in viral maturation.
Figures

Similar articles
-
Comparison of human immunodeficiency virus type 1 Pr55(Gag) and Pr160(Gag-pol) processing intermediates that accumulate in primary and transformed cells treated with peptidic and nonpeptidic protease inhibitors.Antimicrob Agents Chemother. 2000 May;44(5):1397-403. doi: 10.1128/AAC.44.5.1397-1403.2000. Antimicrob Agents Chemother. 2000. PMID: 10770790 Free PMC article.
-
Darunavir: a review of its use in the management of HIV infection in adults.Drugs. 2009;69(4):477-503. doi: 10.2165/00003495-200969040-00007. Drugs. 2009. PMID: 19323590 Review.
-
In vivo processing of Pr160gag-pol from human immunodeficiency virus type 1 (HIV) in acutely infected, cultured human T-lymphocytes.Virology. 1995 Dec 20;214(2):624-7. doi: 10.1006/viro.1995.0074. Virology. 1995. PMID: 8553565
-
Natural variation in HIV-1 protease, Gag p7 and p6, and protease cleavage sites within gag/pol polyproteins: amino acid substitutions in the absence of protease inhibitors in mothers and children infected by human immunodeficiency virus type 1.Virology. 1996 May 15;219(2):407-16. doi: 10.1006/viro.1996.0266. Virology. 1996. PMID: 8638406
-
Human Immunodeficiency Virus Gag and protease: partners in resistance.Retrovirology. 2012 Aug 6;9:63. doi: 10.1186/1742-4690-9-63. Retrovirology. 2012. PMID: 22867298 Free PMC article. Review.
Cited by
-
Recent Updates on Viral Oncogenesis: Available Preventive and Therapeutic Entities.Mol Pharm. 2023 Aug 7;20(8):3698-3740. doi: 10.1021/acs.molpharmaceut.2c01080. Epub 2023 Jul 24. Mol Pharm. 2023. PMID: 37486263 Free PMC article. Review.
-
Identification of potential antivirals against SARS-CoV-2 using virtual screening method.Inform Med Unlocked. 2021;23:100531. doi: 10.1016/j.imu.2021.100531. Epub 2021 Feb 10. Inform Med Unlocked. 2021. PMID: 33594342 Free PMC article.
-
COVID-19 therapeutics: Clinical application of repurposed drugs and futuristic strategies for target-based drug discovery.Genes Dis. 2023 Jul;10(4):1402-1428. doi: 10.1016/j.gendis.2022.12.019. Epub 2023 Apr 7. Genes Dis. 2023. PMID: 37334160 Free PMC article. Review.
-
Viral proteases as therapeutic targets.Mol Aspects Med. 2022 Dec;88:101159. doi: 10.1016/j.mam.2022.101159. Epub 2022 Nov 29. Mol Aspects Med. 2022. PMID: 36459838 Free PMC article. Review.
-
Highly resistant HIV-1 proteases and strategies for their inhibition.Future Med Chem. 2015;7(8):1023-38. doi: 10.4155/fmc.15.44. Future Med Chem. 2015. PMID: 26062399 Free PMC article. Review.
References
-
- Bickel M, et al. 2009. Once-daily treatment with saquinavir mesylate (2000 mg) and ritonavir (100 mg) together with a fixed-dose combination of abacavir/lamivudine (600/300 mg) or tenofovir/emtricitabine (245/200 mg) in HIV-1-infected patients. J. Antimicrob. Chemother. 64:1260–1264 - PubMed
-
- Bragman K. 1996. Saquinavir: an HIV proteinase inhibitor. Adv. Exp. Med. Biol. 394:305–317 - PubMed
-
- Burlet S, et al. 2005. Prospects for the resistance to HIV protease inhibitors: current drug design approaches and perspectives. Curr. Pharm. Des. 11:3077–3090 - PubMed
-
- Cressey TR, et al. 2005. Low-doses of indinavir boosted with ritonavir in HIV-infected Thai patients: pharmacokinetics, efficacy and tolerability. J. Antimicrob. Chemother. 55:1041–1044 - PubMed
-
- Croom K. F., Keam. S. J. 2005. Tipranavir: a ritonavir-boosted protease inhibitor. Drugs 65:1669–1677; discussion, 1678–1679 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

