Increased vulnerability of the brain norepinephrine system of females to corticotropin-releasing factor overexpression
- PMID: 22508464
- PMCID: PMC3402704
- DOI: 10.1038/mp.2012.24
Increased vulnerability of the brain norepinephrine system of females to corticotropin-releasing factor overexpression
Abstract
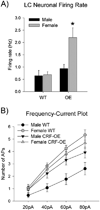
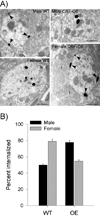
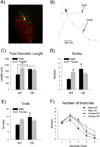
Stress-related psychiatric disorders are more prevalent in women than men. As hypersecretion of the stress neuromediator, corticotropin-releasing factor (CRF) has been implicated in these disorders, sex differences in CRF sensitivity could underlie this disparity. Hyperarousal is a core symptom that is shared by stress-related disorders and this has been attributed to CRF regulation of the locus ceruleus (LC)-norepinephrine arousal system. We recently identified sex differences in CRF(1) receptor (CRF(1)) signaling and trafficking that render LC neurons of female rats more sensitive to CRF and potentially less able to adapt to excess CRF compared with male rats. The present study used a genetic model of CRF overexpression to test the hypothesis that females would be more vulnerable to LC dysregulation by conditions of excess CRF. In both male and female CRF overexpressing (CRF-OE) mice, the LC was more densely innervated by CRF compared with wild-type controls. Despite the equally dense CRF innervation of the LC in male and female CRF-OE mice, LC discharge rates recorded in slices in vitro were selectively elevated in female CRF-OE mice. Immunoelectron microscopy revealed that this sex difference resulted from differential CRF(1) trafficking. In male CRF-OE mice, CRF(1) immunolabeling was prominent in the cytoplasm of LC neurons, indicative of internalization, a process that would protect cells from excessive CRF. However, in female CRF-OE mice, CRF(1) labeling was more prominent on the plasma membrane, suggesting that the compensatory response of internalization was compromised. Together, the findings suggest that the LC-norepinephrine system of females will be particularly affected by conditions resulting in elevated CRF because of differences in receptor trafficking. As excessive LC activation has been implicated in the arousal components of stress-related psychiatric disorders, this may be a cellular mechanism that contributes to the increased incidence of these disorders in females.
Figures

References
-
- Fava M, Kendler KS. Major depressive disorder. Neuron. 2000;28(2):335–341. - PubMed
-
- Keane TM, Marshall AD, Taft CT. Posttraumatic stress disorder: etiology, epidemiology, and treatment outcome. Annu Rev Clin Psychol. 2006;2:161–197. - PubMed
-
- Kendler KS, Kessler RC, Walters EE, MacLean C, Neale MC, Heath AC, et al. Stressful life events, genetic liability, and onset of an episode of major depression in women. Am J Psychiatry. 1995;152(6):833–842. - PubMed
-
- Kessler RC, McGonagle KA, Nelson CB, Hughes M, Swartz M, Blazer DG. Sex and depression in the National Comorbidity Survey. II: Cohort effects. J Affect Disord. 1994;30(1):15–26. - PubMed
-
- Kessler RC. Epidemiology of women and depression. J Affect Disord. 2003;74(1):5–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R00 MH092438/MH/NIMH NIH HHS/United States
- P01 MH048125/MH/NIMH NIH HHS/United States
- MH089800/MH/NIMH NIH HHS/United States
- RC1 MH089800/MH/NIMH NIH HHS/United States
- R21 MH099488/MH/NIMH NIH HHS/United States
- MH092438/MH/NIMH NIH HHS/United States
- MH084423/MH/NIMH NIH HHS/United States
- MH0754047/MH/NIMH NIH HHS/United States
- R01 MH040008/MH/NIMH NIH HHS/United States
- R01 MH075047/MH/NIMH NIH HHS/United States
- K99 MH092438/MH/NIMH NIH HHS/United States
- F32 MH084423/MH/NIMH NIH HHS/United States
- MH40008/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

