The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies
- PMID: 22508478
- PMCID: PMC3819728
- DOI: 10.1038/onc.2012.130
The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies
Abstract
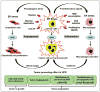
Cancer progression is characterized by rapidly proliferating cancer cells that are in need of increased protein synthesis. Therefore, enhanced endoplasmic reticulum (ER) activity is required to facilitate the folding, assembly and transportation of membrane and secretory proteins. These functions are carried out by ER chaperones. It is now becoming clear that the ER chaperones have critical functions outside of simply facilitating protein folding. For example, cancer progression requires glucose regulated protein (GRP) 78 for cancer cell survival and proliferation, as well as angiogenesis in the microenvironment. GRP78 can translocate to the cell surface acting as a receptor regulating oncogenic signaling and cell viability. Calreticulin, another ER chaperone, can translocate to the cell surface of apoptotic cancer cells and induce immunogenic cancer cell death and antitumor responses in vivo. Tumor-secreted GRP94 has been shown to elicit antitumor immune responses when used as antitumor vaccines. Protein disulfide isomerase is another ER chaperone that demonstrates pro-oncogenic and pro-survival functions. Because of intrinsic alterations of cellular metabolism and extrinsic factors in the tumor microenvironment, cancer cells are under ER stress, and they respond to this stress by activating the unfolded protein response (UPR). Depending on the severity and duration of ER stress, the signaling branches of the UPR can activate adaptive and pro-survival signals, or induce apoptotic cell death. The protein kinase RNA-like ER kinase signaling branch of the UPR has a dual role in cancer proliferation and survival, and is also required for ER stress-induced autophagy. The activation of the inositol-requiring kinase 1α branch promotes tumorigenesis, cancer cell survival and regulates tumor invasion. In summary, perturbance of ER homeostasis has critical roles in tumorigenesis, and therapeutic modulation of ER chaperones and/or UPR components presents potential antitumor treatments.
Conflict of interest statement
None
Figures


References
-
- Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol. 2004;14:20–28. - PubMed
-
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. - PubMed
-
- Wu J, Kaufman RJ. From acute ER stress to physiological roles of the unfolded protein response. Cell Death Differ. 2006;13:374–384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

