Development of viral nanoparticles for efficient intracellular delivery
- PMID: 22508503
- PMCID: PMC3563001
- DOI: 10.1039/c2nr30366c
Development of viral nanoparticles for efficient intracellular delivery
Abstract
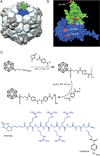
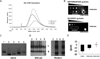
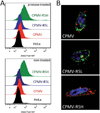
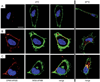
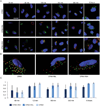
Viral nanoparticles (VNPs) based on plant viruses such as Cowpea mosaic virus (CPMV) can be used for a broad range of biomedical applications because they present a robust scaffold that allows functionalization by chemical conjugation and genetic modification, thereby offering an efficient drug delivery platform that can target specific cells and tissues. VNPs such as CPMV show natural affinity to cells; however, cellular uptake is inefficient. Here we show that chemical modification of the CPMV surface with a highly reactive, specific and UV-traceable hydrazone linker allows bioconjugation of polyarginine (R5) cell penetrating peptides (CPPs), which can overcome these limitations. The resulting CPMV-R5 particles were taken up into a human cervical cancer cell line (HeLa) more efficiently than native particles. Uptake efficiency was dependent on the density of R5 peptides on the surface of the VNP; particles displaying 40 R5 peptides per CPMV (denoted as CPMV-R5H) interact strongly with the plasma membrane and are taken up into the cells via an energy-dependent mechanism whereas particles displaying 10 R5 peptides per CPMV (CPMV-R5L) are only slowly taken up. The fate of CPMV-R5 versus native CPMV particles within cells was evaluated in a co-localization time course study. It was indicated that the intracellular localization of CPMV-R5 and CPMV differs; CPMV remains trapped in Lamp-1 positive endolysosomes over long time frames; in contrast, 30-50% of the CPMV-R5 particles transitioned from the endosome into other cellular vesicles or compartments. Our data provide the groundwork for the development of efficient drug delivery formulations based on CPMV-R5.
Figures
References
-
- Steinmetz NF. Nanomedicine. 2010;349 - PMC - PubMed
- Huo Q, Liu J, Wang LQ, Jiang Y, Lambert TN, Fang E. J. Am. Chem. Soc. 2006;128:6447. - PubMed
- Alexiou C, Schmid RJ, Jurgons R, Kremer M, Wanner G, Bergemann C, Huenges E, Nawroth T, Arnold W, Parak FG. Eur. Biophys. J. 2006;35:446. - PubMed
- Dutta T, Agashe HB, Garg M, Balakrishnan P, Kabra M, Jain NK. J. Drug Targeting. 2007;15:89. - PubMed
- Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Clin. Pharmacol. Ther. 2008;83:761. - PubMed
-
- Jain KK. Med. Princ. Pract. 2008;17:89. - PubMed
-
- Mohanraj V, Chen Y. J. Pharm. Res. 2006;5:561.
-
- Razis ED, Fountzilas G. Ann. Oncol. 2001;12:593. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

