Human telomeres replicate using chromosome-specific, rather than universal, replication programs
- PMID: 22508510
- PMCID: PMC3328383
- DOI: 10.1083/jcb.201112083
Human telomeres replicate using chromosome-specific, rather than universal, replication programs
Abstract
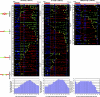
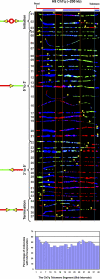
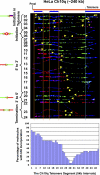
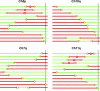
Telomeric and adjacent subtelomeric heterochromatin pose significant challenges to the DNA replication machinery. Little is known about how replication progresses through these regions in human cells. Using single molecule analysis of replicated DNA (SMARD), we delineate the replication programs-i.e., origin distribution, termination site location, and fork rate and direction-of specific telomeres/subtelomeres of individual human chromosomes in two embryonic stem (ES) cell lines and two primary somatic cell types. We observe that replication can initiate within human telomere repeats but was most frequently accomplished by replisomes originating in the subtelomere. No major delay or pausing in fork progression was detected that might lead to telomere/subtelomere fragility. In addition, telomeres from different chromosomes from the same cell type displayed chromosome-specific replication programs rather than a universal program. Importantly, although there was some variation in the replication program of the same telomere in different cell types, the basic features of the program of a specific chromosome end appear to be conserved.
Figures



Similar articles
-
Genome-wide Control of Heterochromatin Replication by the Telomere Capping Protein TRF2.Mol Cell. 2018 May 3;70(3):449-461.e5. doi: 10.1016/j.molcel.2018.03.036. Mol Cell. 2018. PMID: 29727617
-
Replication Timing of Human Telomeres is Conserved during Immortalization and Influenced by Respective Subtelomeres.Sci Rep. 2016 Sep 2;6:32510. doi: 10.1038/srep32510. Sci Rep. 2016. PMID: 27587191 Free PMC article.
-
Replicating through telomeres: a means to an end.Trends Biochem Sci. 2015 Sep;40(9):504-15. doi: 10.1016/j.tibs.2015.06.003. Epub 2015 Jul 15. Trends Biochem Sci. 2015. PMID: 26188776 Review.
-
Anatomy and dynamics of DNA replication fork movement in yeast telomeric regions.Mol Cell Biol. 2004 May;24(9):4019-31. doi: 10.1128/MCB.24.9.4019-4031.2004. Mol Cell Biol. 2004. PMID: 15082794 Free PMC article.
-
Resolving Roadblocks to Telomere Replication.Methods Mol Biol. 2019;1999:31-57. doi: 10.1007/978-1-4939-9500-4_2. Methods Mol Biol. 2019. PMID: 31127568 Review.
Cited by
-
Early telomerase inactivation accelerates aging independently of telomere length.Cell. 2015 Feb 26;160(5):928-939. doi: 10.1016/j.cell.2015.02.002. Cell. 2015. PMID: 25723167 Free PMC article.
-
BLM helicase unwinds lagging strand substrates to assemble the ALT telomere damage response.Mol Cell. 2024 May 2;84(9):1684-1698.e9. doi: 10.1016/j.molcel.2024.03.011. Epub 2024 Apr 8. Mol Cell. 2024. PMID: 38593805 Free PMC article.
-
Consequences of telomere replication failure: the other end-replication problem.Trends Biochem Sci. 2022 Jun;47(6):506-517. doi: 10.1016/j.tibs.2022.03.013. Epub 2022 Apr 16. Trends Biochem Sci. 2022. PMID: 35440402 Free PMC article. Review.
-
Highlighting vulnerabilities in the alternative lengthening of telomeres pathway.Curr Opin Pharmacol. 2023 Jun;70:102380. doi: 10.1016/j.coph.2023.102380. Epub 2023 May 5. Curr Opin Pharmacol. 2023. PMID: 37149932 Free PMC article. Review.
-
DNA Replication Origins and Fork Progression at Mammalian Telomeres.Genes (Basel). 2017 Mar 28;8(4):112. doi: 10.3390/genes8040112. Genes (Basel). 2017. PMID: 28350373 Free PMC article. Review.
References
-
- Arnoult N., Schluth-Bolard C., Letessier A., Drascovic I., Bouarich-Bourimi R., Campisi J., Kim S.H., Boussouar A., Ottaviani A., Magdinier F., et al. 2010. Replication timing of human telomeres is chromosome arm-specific, influenced by subtelomeric structures and connected to nuclear localization. PLoS Genet. 6:e1000920 10.1371/journal.pgen.1000920 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

