The ubiquitin ligase mLin41 temporally promotes neural progenitor cell maintenance through FGF signaling
- PMID: 22508726
- PMCID: PMC3337455
- DOI: 10.1101/gad.187641.112
The ubiquitin ligase mLin41 temporally promotes neural progenitor cell maintenance through FGF signaling
Erratum in
- Genes Dev. 2012 Jun 15;26(12):1392
Abstract
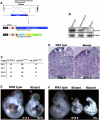
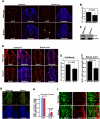
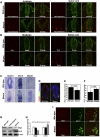
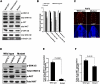
How self-renewal versus differentiation of neural progenitor cells is temporally controlled during early development remains ill-defined. We show that mouse Lin41 (mLin41) is highly expressed in neural progenitor cells and its expression declines during neural differentiation. Loss of mLin41 function in mice causes reduced proliferation and premature differentiation of embryonic neural progenitor cells. mLin41 was recently implicated as the E3 ubiquitin ligase that mediates degradation of Argonaute 2 (AGO2), a key effector of the microRNA pathway. However, our mechanistic studies of neural progenitor cells indicate mLin41 is not required for AGO2 ubiquitination or stability. Instead, mLin41-deficient neural progenitors exhibit hyposensitivity for fibroblast growth factor (FGF) signaling. We show that mLin41 promotes FGF signaling by directly binding to and enhancing the stability of Shc SH2-binding protein 1 (SHCBP1) and that SHCBP1 is an important component of FGF signaling in neural progenitor cells. Thus, mLin41 acts as a temporal regulator to promote neural progenitor cell maintenance, not via the regulation of AGO2 stability, but through FGF signaling.
Figures




References
-
- Ayala R, Shu T, Tsai LH 2007. Trekking across the brain: The journey of neuronal migration. Cell 128: 29–43 - PubMed
-
- Bertrand N, Castro DS, Guillemot F 2002. Proneural genes and the specification of neural cell types. Nature reviews 3: 517–530 - PubMed
-
- Bielas S, Higginbotham H, Koizumi H, Tanaka T, Gleeson JG 2004. Cortical neuronal migration mutants suggest separate but intersecting pathways. Annu Rev Cell Dev Biol 20: 593–618 - PubMed
-
- Briscoe J, Pierani A, Jessell TM, Ericson J 2000. A homeodomain protein code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell 101: 435–445 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
