Multidimensional regulation of gene expression in the C. elegans embryo
- PMID: 22508763
- PMCID: PMC3396369
- DOI: 10.1101/gr.131920.111
Multidimensional regulation of gene expression in the C. elegans embryo
Abstract
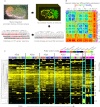
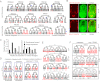
How cells adopt different expression patterns is a fundamental question of developmental biology. We quantitatively measured reporter expression of 127 genes, primarily transcription factors, in every cell and with high temporal resolution in C. elegans embryos. Embryonic cells are highly distinct in their gene expression; expression of the 127 genes studied here can distinguish nearly all pairs of cells, even between cells of the same tissue type. We observed recurrent lineage-regulated expression patterns for many genes in diverse contexts. These patterns are regulated in part by the TCF-LEF transcription factor POP-1. Other genes' reporters exhibited patterns correlated with tissue, position, and left-right asymmetry. Sequential patterns both within tissues and series of sublineages suggest regulatory pathways. Expression patterns often differ between embryonic and larval stages for the same genes, emphasizing the importance of profiling expression in different stages. This work greatly expands the number of genes in each of these categories and provides the first large-scale, digitally based, cellular resolution compendium of gene expression dynamics in live animals. The resulting data sets will be a useful resource for future research.
Figures



References
-
- Ahringer J 1996. Posterior patterning by the Caenorhabditis elegans even-skipped homolog vab-7. Genes Dev 10: 1120–1130 - PubMed
-
- Baugh LR, Hill AA, Claggett JM, Hill-Harfe K, Wen JC, Slonim DK, Brown EL, Hunter CP 2005. The homeodomain protein PAL-1 specifies a lineage-specific regulatory network in the C. elegans embryo. Development 132: 1843–1854 - PubMed
-
- Baumeister R, Liu Y, Ruvkun G 1996. Lineage-specific regulators couple cell lineage asymmetry to the transcription of the Caenorhabditis elegans POU gene unc-86 during neurogenesis. Genes Dev 10: 1395–1410 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
