Loss of meningococcal PilU delays microcolony formation and attenuates virulence in vivo
- PMID: 22508857
- PMCID: PMC3416451
- DOI: 10.1128/IAI.06354-11
Loss of meningococcal PilU delays microcolony formation and attenuates virulence in vivo
Abstract
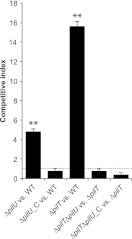
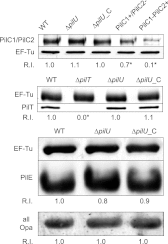
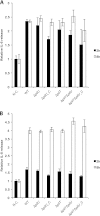
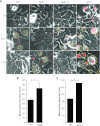
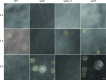
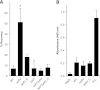
Neisseria meningitidis is a major cause of sepsis and bacterial meningitis worldwide. This bacterium expresses type IV pili (Tfp), which mediate important virulence traits such as the formation of bacterial aggregates, host cell adhesion, twitching motility, and DNA uptake. The meningococcal PilT protein is a hexameric ATPase that mediates pilus retraction. The PilU protein is produced from the pilT-pilU operon and shares a high degree of homology with PilT. The function of PilT in Tfp biology has been studied extensively, whereas the role of PilU remains poorly understood. Here we show that pilU mutants have delayed microcolony formation on host epithelial cells compared to the wild type, indicating that bacterium-bacterium interactions are affected. In normal human serum, the pilU mutant survived at a higher rate than that for wild-type bacteria. However, in a murine model of disease, mice infected with the pilT mutant demonstrated significantly reduced bacterial blood counts and survived at a higher rate than that for mice infected with the wild type. Infection of mice with the pilU mutant resulted in a trend of lower bacteremia, and still a significant increase in survival, than that of the wild type. In conclusion, these data suggest that PilU promotes timely microcolony formation and that both PilU and PilT are required for full bacterial virulence.
Figures

Similar articles
-
Type IV pilus retraction enables sustained bacteremia and plays a key role in the outcome of meningococcal sepsis in a humanized mouse model.PLoS Pathog. 2021 Feb 16;17(2):e1009299. doi: 10.1371/journal.ppat.1009299. eCollection 2021 Feb. PLoS Pathog. 2021. PMID: 33592056 Free PMC article.
-
Pseudomonas aeruginosa gene products PilT and PilU are required for cytotoxicity in vitro and virulence in a mouse model of acute pneumonia.Infect Immun. 1999 Jul;67(7):3625-30. doi: 10.1128/IAI.67.7.3625-3630.1999. Infect Immun. 1999. PMID: 10377148 Free PMC article.
-
The type IV pilus protein PilU functions as a PilT-dependent retraction ATPase.PLoS Genet. 2019 Sep 16;15(9):e1008393. doi: 10.1371/journal.pgen.1008393. eCollection 2019 Sep. PLoS Genet. 2019. PMID: 31525185 Free PMC article.
-
Meningococcal disease: A paradigm of type-IV pilus dependent pathogenesis.Cell Microbiol. 2020 Apr;22(4):e13185. doi: 10.1111/cmi.13185. Cell Microbiol. 2020. PMID: 32185901 Review.
-
An Overview of Neisseria meningitidis.Methods Mol Biol. 2019;1969:1-16. doi: 10.1007/978-1-4939-9202-7_1. Methods Mol Biol. 2019. PMID: 30877666 Review.
Cited by
-
Perturbing the acetylation status of the Type IV pilus retraction motor, PilT, reduces Neisseria gonorrhoeae viability.Mol Microbiol. 2018 Dec;110(5):677-688. doi: 10.1111/mmi.13979. Epub 2018 Oct 28. Mol Microbiol. 2018. PMID: 29719082 Free PMC article.
-
Neisseria meningitidis Polynucleotide Phosphorylase Affects Aggregation, Adhesion, and Virulence.Infect Immun. 2016 Apr 22;84(5):1501-1513. doi: 10.1128/IAI.01463-15. Print 2016 May. Infect Immun. 2016. PMID: 26930706 Free PMC article.
-
Vertebrate and Invertebrate Animal and New In Vitro Models for Studying Neisseria Biology.Pathogens. 2023 May 30;12(6):782. doi: 10.3390/pathogens12060782. Pathogens. 2023. PMID: 37375472 Free PMC article. Review.
-
cjrABC-senB hinders survival of extraintestinal pathogenic E. coli in the bloodstream through triggering complement-mediated killing.J Biomed Sci. 2020 Aug 6;27(1):86. doi: 10.1186/s12929-020-00677-4. J Biomed Sci. 2020. PMID: 32762693 Free PMC article.
-
Type IV pilus retraction enables sustained bacteremia and plays a key role in the outcome of meningococcal sepsis in a humanized mouse model.PLoS Pathog. 2021 Feb 16;17(2):e1009299. doi: 10.1371/journal.ppat.1009299. eCollection 2021 Feb. PLoS Pathog. 2021. PMID: 33592056 Free PMC article.
References
-
- Aas FE, et al. 2006. Neisseria gonorrhoeae type IV pili undergo multisite, hierarchical modifications with phosphoethanolamine and phosphocholine requiring an enzyme structurally related to lipopolysaccharide phosphoethanolamine transferases. J. Biol. Chem. 281:27712–27723 - PubMed
-
- Aas FE, Lovold C, Koomey M. 2002. An inhibitor of DNA binding and uptake events dictates the proficiency of genetic transformation in Neisseria gonorrhoeae: mechanism of action and links to type IV pilus expression. Mol. Microbiol. 46:1441–1450 - PubMed
-
- Chamot-Rooke J, et al. 2011. Posttranslational modification of pili upon cell contact triggers N. meningitidis dissemination. Science 331:778–782 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

