Pro-arrhythmogenic effects of the S140G KCNQ1 mutation in human atrial fibrillation - insights from modelling
- PMID: 22508963
- PMCID: PMC3477754
- DOI: 10.1113/jphysiol.2012.229146
Pro-arrhythmogenic effects of the S140G KCNQ1 mutation in human atrial fibrillation - insights from modelling
Abstract
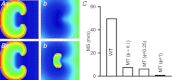
Functional analysis has shown that the missense gain-in-function KCNQ1 S140G mutation associated with familial atrial fibrillation produces an increase of the slow delayed rectifier potassium current (I(Ks)). Through computer modelling, this study investigated mechanisms by which the KCNQ1 S140G mutation promotes and perpetuates atrial fibrillation. In simulations, Courtemanche et al.'s model of human atrial cell action potentials (APs) was modified to incorporate experimental data on changes of I(Ks) induced by the KCNQ1 S140G mutation. The cell models for wild type (WT) and mutant type (MT) I(Ks) were incorporated into homogeneous multicellular 2D and 3D tissue models. Effects of the mutation were quantified on AP profile, AP duration (APD) restitution, effective refractory period (ERP) restitution, and conduction velocity (CV) restitution.Temporal and spatial vulnerabilities of atrial tissue to genesis of re-entry were computed. Dynamic behaviours of re-entrant excitation waves (lifespan (LS), tip meandering patterns and dominant frequency) in 2D and 3D models were characterised. It was shown that the KCNQ1 S140G mutation abbreviated atrial APD and ERP and flattened APD and ERP restitution curves. It reduced atrial CV at low excitation rates, but increased it at high excitation rates that facilitated the conduction of high rate atrial excitation waves. Although it increased slightly tissue temporal vulnerability for initiating re-entry, it reduced markedly the minimal substrate size necessary for sustaining re-entry (increasing the tissue spatial vulnerability). In the 2D and 3D models, the mutation also stabilized and accelerated re-entrant excitation waves, leading to rapid and sustained re-entry. In the 3D model, scroll waves under the mutation condition MT conditions also degenerated into persistent and erratic wavelets, leading to fibrillation. In conclusion, increased I(Ks) due to the KCNQ1 S140G mutation increases atrial susceptibility to arrhythmia due to increased tissue vulnerability, shortened ERP and altered atrial conduction velocity, which, in combination, facilitate initiation and maintenance of re-entrant excitation waves.
Figures






References
-
- Ackerman MJ. The Visible Human Project. J Biocommun. 1991;18:14. - PubMed
-
- Ackerman MJ, Banvard RA. Imaging outcomes from the National Library of Medicine's Visible Human Project. Comput Med Imaging Graph. 2000;24:125–126. - PubMed
-
- Ashcroft FM. From molecule to malady. Nature. 2006;440:440–447. - PubMed
-
- Bellocq C, van Ginneken ACG, Bezzina CR, Alders M, Escande D, Mannens MMAM, Baró I, Wilde AAM. Mutation in the KCNQ1 gene leading to the short QT-interval syndrome. Circulation. 2004;109:2394–2397. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

