Cks1 promotion of S phase entry and proliferation is independent of p27Kip1 suppression
- PMID: 22508990
- PMCID: PMC3434501
- DOI: 10.1128/MCB.06771-11
Cks1 promotion of S phase entry and proliferation is independent of p27Kip1 suppression
Abstract
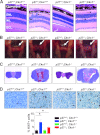
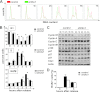
Cks1 is an activator of the SCF(Skp2) ubiquitin ligase complex that targets the cell cycle inhibitor p27(Kip1) for degradation. The loss of Cks1 results in p27(Kip1) accumulation and decreased proliferation and inhibits tumorigenesis. We identify here a function of Cks1 in mammalian cell cycle regulation that is independent of p27(Kip1). Specifically, Cks1(-/-); p27(Kip1-/-) mouse embryonic fibroblasts retain defects in the G(1)-S phase transition that are coupled with decreased Cdk2-associated kinase activity and defects in proliferation that are associated with Cks1 loss. Furthermore, concomitant loss of Cks1 does not rescue the tumor suppressor function of p27(Kip1) that is manifest in various organs of p27(Kip1-/-) mice. In contrast, defects in mitotic entry and premature senescence manifest in Cks1(-/-) cells are p27(Kip1) dependent. Collectively, these findings establish p27(Kip1)-independent functions of Cks1 in regulating the G(1)-S transition.
Figures







References
-
- Aleem E, Kiyokawa H, Kaldis P. 2005. Cdc2-cyclin E complexes regulate the G1/S phase transition. Nat. Cell Biol. 7: 831–836 - PubMed
-
- Arvai AS, Bourne Y, Hickey MJ, Tainer JA. 1995. Crystal structure of the human cell cycle protein CksHs1: single domain fold with similarity to kinase N-lobe domain. J. Mol. Biol. 249: 835–842 - PubMed
-
- Berthet C, Aleem E, Coppola V, Tessarollo L, Kaldis P. 2003. Cdk2 knockout mice are viable. Curr. Biol. 13: 1775–1785 - PubMed
-
- Bhattacharya S, et al. 2003. SKP2 associates with p130 and accelerates p130 ubiquitylation and degradation in human cells. Oncogene 22: 2443–2451 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
