Pyruvate kinase M2 promotes de novo serine synthesis to sustain mTORC1 activity and cell proliferation
- PMID: 22509023
- PMCID: PMC3345000
- DOI: 10.1073/pnas.1204176109
Pyruvate kinase M2 promotes de novo serine synthesis to sustain mTORC1 activity and cell proliferation
Abstract
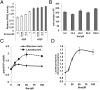
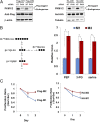
Despite the fact that most cancer cells display high glycolytic activity, cancer cells selectively express the less active M2 isoform of pyruvate kinase (PKM2). Here we demonstrate that PKM2 expression makes a critical regulatory contribution to the serine synthetic pathway. In the absence of serine, an allosteric activator of PKM2, glycolytic efflux to lactate is significantly reduced in PKM2-expressing cells. This inhibition of PKM2 results in the accumulation of glycolytic intermediates that feed into serine synthesis. As a consequence, PKM2-expressing cells can maintain mammalian target of rapamycin complex 1 activity and proliferate in serine-depleted medium, but PKM1-expressing cells cannot. Cellular detection of serine depletion depends on general control nonderepressible 2 kinase-activating transcription factor 4 (GCN2-ATF4) pathway activation and results in increased expression of enzymes required for serine synthesis from the accumulating glycolytic precursors. These findings suggest that tumor cells use serine-dependent regulation of PKM2 and GCN2 to modulate the flux of glycolytic intermediates in support of cell proliferation.
Conflict of interest statement
Conflict of interest statement: C.B.T. is a cofounder of and consultant at Agios Pharmaceuticals.
Figures



References
-
- Carbonell J, Feliu JE, Marco R, Sols A. Pyruvate kinase. Classes of regulatory isoenzymes in mammalian tissues. Eur J Biochem. 1973;37(1):148–156. - PubMed
-
- Rodriguez-Horche P, Luque J, Perez-Artes E, Pineda M, Pinilla M. Comparative kinetic behaviour and regulation by fructose-1,6-bisphosphate and ATP of pyruvate kinase from erythrocytes, reticulocytes and bone marrow cells. Comp Biochem Physiol B. 1987;87:553–557. - PubMed
-
- Kahn A, Marie J, Boivin P. Pyruvate kinase isozymes in man. II. L type and erythrocyte-type isozymes. Electrofocusing and immunologic studies. Hum Genet. 1976;33:35–46. - PubMed
-
- Marie J, Levin MJ, Simon MP, Kahn A. Genetic and epigenetic control of the pyruvate kinase isozymes in mammals. Isozymes Curr Top Biol Med Res. 1983;7:221–240. - PubMed
-
- Noguchi T, Inoue H, Tanaka T. The M1- and M2-type isozymes of rat pyruvate kinase are produced from the same gene by alternative RNA splicing. J Biol Chem. 1986;261:13807–13812. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

