Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation
- PMID: 22509047
- PMCID: PMC3344960
- DOI: 10.1073/pnas.1202288109
Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation
Abstract
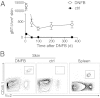
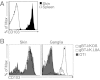
Although circulating memory T cells provide enhanced protection against pathogen challenge, they often fail to do so if infection is localized to peripheral or extralymphoid compartments. In those cases, it is T cells already resident at the site of virus challenge that offer superior immune protection. These tissue-resident memory T (T(RM)) cells are identified by their expression of the α-chain from the integrin α(E)(CD103)β(7), and can exist in disequilibrium with the blood, remaining in the local environment long after peripheral infections subside. In this study, we demonstrate that long-lived intraepithelial CD103(+)CD8(+) T(RM) cells can be generated in the absence of in situ antigen recognition. Local inflammation in skin and mucosa alone resulted in enhanced recruitment of effector populations and their conversion to the T(RM) phenotype. The CD8(+) T(RM) cells lodged in these barrier tissues provided long-lived protection against local challenge with herpes simplex virus in skin and vagina challenge models, and were clearly superior to the circulating memory T-cell cohort. The results demonstrate that peripheral T(RM) cells can be generated and survive in the absence of local antigen presentation and provide a powerful means of achieving immune protection against peripheral infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


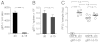

References
-
- Welsh RM, Selin LK, Szomolanyi-Tsuda E. Immunological memory to viral infections. Annu Rev Immunol. 2004;22:711–743. - PubMed
-
- Harty JT, Tvinnereim AR, White DW. CD8+ T cell effector mechanisms in resistance to infection. Annu Rev Immunol. 2000;18:275–308. - PubMed
-
- Bachmann MF, Wolint P, Schwarz K, Oxenius A. Recall proliferation potential of memory CD8+ T cells and antiviral protection. J Immunol. 2005;175:4677–4685. - PubMed
-
- Badovinac VP, Messingham KA, Jabbari A, Haring JS, Harty JT. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nat Med. 2005;11:748–756. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

