HBx activates FasL and mediates HepG2 cell apoptosis through MLK3-MKK7-JNKs signal module
- PMID: 22509080
- PMCID: PMC3319944
- DOI: 10.3748/wjg.v18.i13.1485
HBx activates FasL and mediates HepG2 cell apoptosis through MLK3-MKK7-JNKs signal module
Abstract
Aim: To investigate the possible mechanism by which hepatitis B virus X protein (HBx) mediates apoptosis of HepG2 cells.
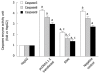
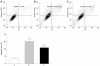
Methods: HBx expression vector pcDNA3.1-X was transfected into HepG2 cells to establish an HBx high-expression cellular model as pcDNA3.1-X transfected group. The pcDNA3.1-X and pSilencer3.1-shHBX (HBx antagonist) were cotransfected into HepG2 cells to establish an HBx low-expression model as RNAi group. Untransfected HepG2 cells and HepG2 cells transfected with negative control plasmid were used as controls. Apoptosis rate, the expression of Fas/FasL signaling pathway-related proteins and the phosphorylation levels of MLK3, MKK7 and JNKs, which are upstream molecules of death receptor pathways and belong to the family of mitogen-activated protein kinases (MAPKs), were measured in each group.
Results: Compared with HepG2 cell group and RNAi group, apoptosis rate, the expression of Fas and FasL proteins, and the activation of MLK3, MKK7 and JNKs were increased in the pcDNA3.1-X transfected group. The activation of JNKs and expression of FasL protein were inhibited in the pcDNA3.1-X transfected group when treated with a known JNK inhibitor, SP600125. When authors treated pcDNA3.1-X transfected group with K252a, a known MLK3 inhibitor, the activation of MLK3, MKK7 and JNKs as well as expression of FasL protein was inhibited. Furthermore, cell apoptosis rate was also significantly declined in the presence of K252a in the pcDNA3.1-X transfected group.
Conclusion: HBx can induce HepG2 cell apoptosis via a novel active MLK3-MKK7-JNKs signaling module to upregulate FasL protein expression.
Keywords: Apoptosis; FasL; HepG2 cell; Hepatitis B virus X protein; MLK3.
Figures






References
-
- Neuveut C, Wei Y, Buendia MA. Mechanisms of HBV-related hepatocarcinogenesis. J Hepatol. 2010;52:594–604. - PubMed
-
- Zhang X, Zhang H, Ye L. Effects of hepatitis B virus X protein on the development of liver cancer. J Lab Clin Med. 2006;147:58–66. - PubMed
-
- Miao J, Chen GG, Chun SY, Lai PP. Hepatitis B virus X protein induces apoptosis in hepatoma cells through inhibiting Bcl-xL expression. Cancer Lett. 2006;236:115–124. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

