Recovery of neurofilament following early monocular deprivation
- PMID: 22509156
- PMCID: PMC3321409
- DOI: 10.3389/fnsys.2012.00022
Recovery of neurofilament following early monocular deprivation
Abstract
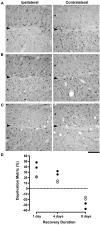
Postnatal development of the mammalian geniculostriate visual pathway is partly guided by visually driven activity. Disruption of normal visual input during certain critical periods can alter the structure of neurons, as well as their connections and functional properties. Within the layers of the dorsal lateral geniculate nucleus (dLGN), a brief early period of monocular deprivation can alter the structure and soma size of neurons within deprived-eye-receiving layers. This modification of structure is accompanied by a marked reduction in labeling for neurofilament protein, a principle component of the stable cytoskeleton. This study examined the extent of neurofilament recovery in monocularly deprived cats that either had their deprived eye opened (binocular recovery), or had the deprivation reversed to the fellow eye (reverse occlusion). The loss of neurofilament and the reduction of soma size caused by monocular deprivation were ameliorated equally and substantially in both recovery conditions after 8 days. The degree to which this recovery was dependent on visually driven activity was examined by placing monocularly deprived animals in complete darkness. Though monocularly deprived animals placed in darkness showed recovery of soma size in deprived layers, the manipulation catalyzed a loss of neurofilament labeling that extended to non-deprived layers as well. Overall, these results indicate that both recovery of soma size and neurofilament labeling is achieved by removal of the competitive disadvantage of the deprived eye. However, while the former occurred even in the absence of visually driven activity, recovery of neurofilament did not. The finding that a period of darkness produced an overall loss of neurofilament throughout the dLGN suggests that this experiential manipulation may cause the visual pathways to revert to an earlier more plastic developmental stage. It is possible that short periods of darkness could be incorporated as a component of therapeutic measures for treatment of deprivation-induced disorders such as amblyopia.
Keywords: cytoskeleton; dark-rearing; lateral geniculate nucleus; monocular deprivation; neurofilament; plasticity; recovery; reverse occlusion.
Figures



Similar articles
-
Binocular eyelid closure promotes anatomical but not behavioral recovery from monocular deprivation.Vision Res. 2015 Sep;114:151-60. doi: 10.1016/j.visres.2014.12.012. Epub 2014 Dec 20. Vision Res. 2015. PMID: 25536470
-
Susceptibility to monocular deprivation following immersion in darkness either late into or beyond the critical period.J Comp Neurol. 2016 Sep 1;524(13):2643-53. doi: 10.1002/cne.23985. Epub 2016 Mar 6. J Comp Neurol. 2016. PMID: 26878686
-
Recovery from the anatomical effects of long-term monocular deprivation in cat lateral geniculate nucleus.J Comp Neurol. 2018 Feb 1;526(2):310-323. doi: 10.1002/cne.24336. Epub 2017 Oct 27. J Comp Neurol. 2018. PMID: 29023717
-
Short periods of darkness fail to restore visual or neural plasticity in adult cats.Vis Neurosci. 2018 Jan;35:E002. doi: 10.1017/S0952523817000335. Vis Neurosci. 2018. PMID: 29905119
-
Cytoskeleton alteration correlates with gross structural plasticity in the cat lateral geniculate nucleus.Vis Neurosci. 2007 Nov-Dec;24(6):775-85. doi: 10.1017/S095252380707068X. Epub 2007 Oct 4. Vis Neurosci. 2007. PMID: 17915043
Cited by
-
Critical periods in amblyopia.Vis Neurosci. 2018 Jan;35:E014. doi: 10.1017/S0952523817000219. Vis Neurosci. 2018. PMID: 29905116 Free PMC article. Review.
-
Dark Rearing Promotes the Recovery of Visual Cortical Responses but Not the Morphology of Geniculocortical Axons in Amblyopic Cat.Front Neural Circuits. 2021 Apr 16;15:637638. doi: 10.3389/fncir.2021.637638. eCollection 2021. Front Neural Circuits. 2021. PMID: 33935657 Free PMC article.
-
Recovery of visual functions in amblyopic animals following brief exposure to total darkness.J Physiol. 2016 Jan 1;594(1):149-67. doi: 10.1113/JP270981. Epub 2015 Nov 15. J Physiol. 2016. PMID: 26449521 Free PMC article.
-
Early monocular deprivation reduces the capacity for neural plasticity in the cat visual system.Cereb Cortex Commun. 2023 Aug 17;4(3):tgad017. doi: 10.1093/texcom/tgad017. eCollection 2023. Cereb Cortex Commun. 2023. PMID: 37675436 Free PMC article.
-
Global processing in amblyopia: a review.Front Psychol. 2014 Jun 17;5:583. doi: 10.3389/fpsyg.2014.00583. eCollection 2014. Front Psychol. 2014. PMID: 24987383 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

