Pharmacokinetics, metabolism and toxicity of carbon nanotubes for biomedical purposes
- PMID: 22509195
- PMCID: PMC3326738
- DOI: 10.7150/thno.3618
Pharmacokinetics, metabolism and toxicity of carbon nanotubes for biomedical purposes
Abstract
Carbon nanotubes (CNTs) have attracted great interest of the nano community and beyond. However, the biomedical applications of CNTs arouse serious concerns for their unknown in vivo consequence, in which the information of pharmacokinetics, metabolism and toxicity of CNTs is essential. In this review, we summarize the updated data of CNTs from the biomedical view. The information shows that surface chemistry is crucial in regulating the in vivo behaviors of CNTs. Among the functionalization methods, PEGylation is the most efficient one to improve the pharmacokinetics and biocompatibility of CNTs. The guiding effects of the pharmacokinetics, metabolism and toxicity information on the biomedical applications of CNTs are discussed.
Keywords: Carbon nanotubes; biomedical applications.; metabolism; pharmacokinetics; toxicity.
Conflict of interest statement
Conflict of Interest: The authors have declared that no conflict of interest exists.
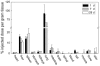
Figures









References
-
- Schnorr JM, Swager TM. Emerging applications of carbon nanotubes. Chem Mater. 2011;23:646–657.
-
- Lu F, Gu L, Meziani MJ. et al. Advances in bioapplications of carbon nanotubes. Adv Mater. 2009;21:139–152.
-
- Thakare VS, Das M, Jain AK. et al. Carbon nanotubes in cancer theragnosis. Nanomedicine. 2010;5:1277–1301. - PubMed
-
- Vashist SK, Zheng D, Pastorin G. et al. Delivery of drugs and biomolecules using carbon nanotubes. Carbon. 2011;49:4077–4097.
-
- Moon HK, Lee SH, Choi HC. In vivo near-infrared mediated tumor destruction by photothermal effect of carbon nanotubes. ACS Nano. 2009;3:3707–3713. - PubMed
LinkOut - more resources
Full Text Sources

