Synthesis of heteroglycoclusters by using orthogonal chemoselective ligations
- PMID: 22509212
- PMCID: PMC3326620
- DOI: 10.3762/bjoc.8.47
Synthesis of heteroglycoclusters by using orthogonal chemoselective ligations
Abstract
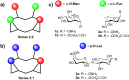
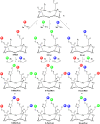
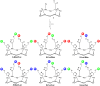
Synthetic heteroglycoclusters are being subjected to increasing interest due to their potential to serve as selective ligands for carbohydrate-binding proteins. In this paper, we describe an expedient strategy to prepare cyclopeptides displaying well-defined distributions and combinations of carbohydrates. By using both oxime ligation and copper(I)-catalyzed alkyne-azide cycloaddition, two series of compounds bearing binary combinations of αMan, αFuc or βLac in an overall tetravalent presentation, and either 2:2 or 3:1 relative proportions, have been prepared.
Keywords: chemoselective ligation; click chemistry; cyclopeptide; heteroglycocluster; oxime.
Figures
Similar articles
-
Orthogonal dual thiol-chloroacetyl and thiol-ene couplings for the sequential one-pot assembly of heteroglycoclusters.Beilstein J Org Chem. 2014 Jul 8;10:1557-63. doi: 10.3762/bjoc.10.160. eCollection 2014. Beilstein J Org Chem. 2014. PMID: 25161711 Free PMC article.
-
Synthesis of a New Series of Sialylated Homo- and Heterovalent Glycoclusters by using Orthogonal Ligations.ChemistryOpen. 2016 Jul 22;5(5):477-484. doi: 10.1002/open.201600062. eCollection 2016 Oct. ChemistryOpen. 2016. PMID: 27777841 Free PMC article.
-
One-pot approach to well-defined biomolecular assemblies by orthogonal chemoselective ligations.Angew Chem Int Ed Engl. 2009;48(14):2576-9. doi: 10.1002/anie.200806223. Angew Chem Int Ed Engl. 2009. PMID: 19229906
-
Application of Chemoselective Ligation in Biosensing.Crit Rev Anal Chem. 2022;52(1):170-193. doi: 10.1080/10408347.2020.1791044. Epub 2020 Jul 14. Crit Rev Anal Chem. 2022. PMID: 32662656 Review.
-
Efficient construction of therapeutics, bioconjugates, biomaterials and bioactive surfaces using azide-alkyne "click" chemistry.Adv Drug Deliv Rev. 2008 Jun 10;60(9):958-70. doi: 10.1016/j.addr.2008.02.004. Epub 2008 Mar 4. Adv Drug Deliv Rev. 2008. PMID: 18406491 Review.
Cited by
-
Heteroglycoclusters With Dual Nanomolar Affinities for the Lectins LecA and LecB From Pseudomonas aeruginosa.Front Chem. 2019 Oct 2;7:666. doi: 10.3389/fchem.2019.00666. eCollection 2019. Front Chem. 2019. PMID: 31632954 Free PMC article.
-
Microarray Analysis of Oligosaccharide-Mediated Multivalent Carbohydrate-Protein Interactions and Their Heterogeneity.Chembiochem. 2018 Mar 25:10.1002/cbic.201800037. doi: 10.1002/cbic.201800037. Online ahead of print. Chembiochem. 2018. PMID: 29575424 Free PMC article.
-
Orthogonal dual thiol-chloroacetyl and thiol-ene couplings for the sequential one-pot assembly of heteroglycoclusters.Beilstein J Org Chem. 2014 Jul 8;10:1557-63. doi: 10.3762/bjoc.10.160. eCollection 2014. Beilstein J Org Chem. 2014. PMID: 25161711 Free PMC article.
-
Multivalent glycocyclopeptides: conjugation methods and biological applications.Chem Soc Rev. 2022 Oct 17;51(20):8756-8783. doi: 10.1039/d2cs00640e. Chem Soc Rev. 2022. PMID: 36193815 Free PMC article. Review.
-
Synthesis of a New Series of Sialylated Homo- and Heterovalent Glycoclusters by using Orthogonal Ligations.ChemistryOpen. 2016 Jul 22;5(5):477-484. doi: 10.1002/open.201600062. eCollection 2016 Oct. ChemistryOpen. 2016. PMID: 27777841 Free PMC article.
References
-
- Renaudet O, Spinelli N, editors. Synthesis and biological applications of glycoconjugates. U.A.E.: Bentham Science Publishers Ltd.; 2011.
LinkOut - more resources
Full Text Sources
