Synthesis of heteroglycoclusters by using orthogonal chemoselective ligations
- PMID: 22509212
- PMCID: PMC3326620
- DOI: 10.3762/bjoc.8.47
Synthesis of heteroglycoclusters by using orthogonal chemoselective ligations
Abstract
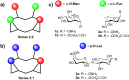
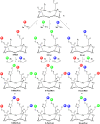
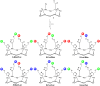
Synthetic heteroglycoclusters are being subjected to increasing interest due to their potential to serve as selective ligands for carbohydrate-binding proteins. In this paper, we describe an expedient strategy to prepare cyclopeptides displaying well-defined distributions and combinations of carbohydrates. By using both oxime ligation and copper(I)-catalyzed alkyne-azide cycloaddition, two series of compounds bearing binary combinations of αMan, αFuc or βLac in an overall tetravalent presentation, and either 2:2 or 3:1 relative proportions, have been prepared.
Keywords: chemoselective ligation; click chemistry; cyclopeptide; heteroglycocluster; oxime.
Figures
References
-
- Renaudet O, Spinelli N, editors. Synthesis and biological applications of glycoconjugates. U.A.E.: Bentham Science Publishers Ltd.; 2011.
LinkOut - more resources
Full Text Sources
