Discovery of practical production processes for arylsulfur pentafluorides and their higher homologues, bis- and tris(sulfur pentafluorides): Beginning of a new era of "super-trifluoromethyl" arene chemistry and its industry
- PMID: 22509218
- PMCID: PMC3326626
- DOI: 10.3762/bjoc.8.53
Discovery of practical production processes for arylsulfur pentafluorides and their higher homologues, bis- and tris(sulfur pentafluorides): Beginning of a new era of "super-trifluoromethyl" arene chemistry and its industry
Abstract
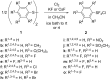
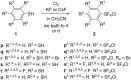
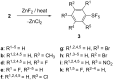
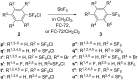
Various arylsulfur pentafluorides, ArSF(5), have long been desired in both academic and industrial areas, and ArSF(5) compounds have attracted considerable interest in many areas such as medicines, agrochemicals, and other new materials, since the highly stable SF(5) group is considered a "super-trifluoromethyl group" due to its significantly higher electronegativity and lipophilicity. This article describes the first practical method for the production of various arylsulfur pentafluorides and their higher homologues, bis- and tris(sulfur pentafluorides), from the corresponding diaryl disulfides or aryl thiols. The method consists of two steps: (Step 1) treatment of a diaryl disulfide or an aryl thiol with chlorine in the presence of an alkali metal fluoride, and (step 2) treatment of the resulting arylsulfur chlorotetrafluoride with a fluoride source, such as ZnF(2), HF, and Sb(III/V) fluorides. The intermediate arylsulfur chlorotetrafluorides were isolated by distillation or recrystallization and characterized. The aspects of these new reactions are revealed and reaction mechanisms are discussed. As the method offers considerable improvement over previous methods in cost, yield, practicality, applicability, and large-scale production, the new processes described here can be employed as the first practical methods for the economical production of various arylsulfur pentafluorides and their higher homologues, which could then open up a new era of "super-trifluoromethyl" arene chemistry and its applications in many areas.
Keywords: arylsulfur chlorotetrafluoride; arylsulfur pentafluoride; pentafluorosulfanyl; sulfur pentafluoride; super-trifluoromethyl.
Figures
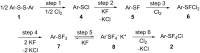
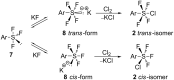
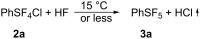

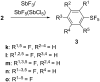
Similar articles
-
Silver-induced self-immolative Cl-F exchange fluorination of arylsulfur chlorotetrafluorides: synthesis of arylsulfur pentafluorides.Chem Commun (Camb). 2017 Nov 28;53(95):12738-12741. doi: 10.1039/c7cc07222h. Chem Commun (Camb). 2017. PMID: 29038817
-
Synthesis and characterization of 2-pyridylsulfur pentafluorides.Angew Chem Int Ed Engl. 2015 Jan 2;54(1):280-4. doi: 10.1002/anie.201409990. Epub 2014 Nov 6. Angew Chem Int Ed Engl. 2015. PMID: 25376393
-
Synthesis of aliphatic sulfur pentafluorides by oxidation of SF₅-containing anisole, phenols, and anilines.J Org Chem. 2014 Sep 19;79(18):8906-11. doi: 10.1021/jo501562z. Epub 2014 Aug 27. J Org Chem. 2014. PMID: 25137015
-
(Hetero)aryl-SVI Fluorides: Synthetic Development and Opportunities.Angew Chem Int Ed Engl. 2022 Jun 7;61(23):e202200904. doi: 10.1002/anie.202200904. Epub 2022 Apr 27. Angew Chem Int Ed Engl. 2022. PMID: 35303387 Free PMC article. Review.
-
[Development of Bioactive Molecules Bearing Hexacoordinated Sulfur Fluoride].Yakugaku Zasshi. 2023;143(5):429-434. doi: 10.1248/yakushi.22-00205-2. Yakugaku Zasshi. 2023. PMID: 37121758 Review. Japanese.
Cited by
-
Oxidative Fluorination of Selenium and Tellurium Compounds using a Thermally Stable Phosphonium SF5 - Salt Accessible from SF6.Angew Chem Int Ed Engl. 2022 Oct 17;61(42):e202209067. doi: 10.1002/anie.202209067. Epub 2022 Sep 12. Angew Chem Int Ed Engl. 2022. PMID: 36018610 Free PMC article.
-
Fluoroalkylated hypervalent sulfur fluorides: radical addition of arylchlorotetrafluoro-λ6-sulfanes to tetrafluoroethylene.Chem Sci. 2023 Oct 19;14(43):12379-12385. doi: 10.1039/d3sc04837c. eCollection 2023 Nov 8. Chem Sci. 2023. PMID: 37969576 Free PMC article.
-
Pentafluorosulfanyl-functionalised BODIPY push-pull dyes for p-type dye-sensitized solar cells.Sustain Energy Fuels. 2023 Feb 21;7(6):1494-1501. doi: 10.1039/d2se00977c. eCollection 2023 Mar 14. Sustain Energy Fuels. 2023. PMID: 36936698 Free PMC article.
-
Synthesis of Aryl Propionamide Scaffold Containing a Pentafluorosulfanyl Moiety as SARMs.Molecules. 2019 Nov 20;24(23):4227. doi: 10.3390/molecules24234227. Molecules. 2019. PMID: 31757115 Free PMC article.
-
Pyridine-promoted dediazoniation of aryldiazonium tetrafluoroborates: Application to the synthesis of SF5-substituted phenylboronic esters and iodobenzenes.Beilstein J Org Chem. 2015 Aug 26;11:1494-502. doi: 10.3762/bjoc.11.162. eCollection 2015. Beilstein J Org Chem. 2015. PMID: 26425206 Free PMC article.
References
-
- Kirsch P. Modern Fluoroorganic Chemistry. Weinheim, Germany: Wiley-VCH; 2004. p. 151. - DOI
-
- Sheppard W A. J Am Chem Soc. 1962;84:3072–3076. doi: 10.1021/ja00875a007. - DOI
-
- Sheppard W A. J Am Chem Soc. 1962;84:3064–3072. doi: 10.1021/ja00875a006. - DOI
-
- Hansch C, Muir R M, Fujita T, Maloney P P, Geiger F, Streich M. J Am Chem Soc. 1963;85:2817–2824. doi: 10.1021/ja00901a033. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
