The role of natural killer (NK) cells and NK cell receptor polymorphisms in the assessment of HIV-1 neutralization
- PMID: 22509241
- PMCID: PMC3324450
- DOI: 10.1371/journal.pone.0029454
The role of natural killer (NK) cells and NK cell receptor polymorphisms in the assessment of HIV-1 neutralization
Abstract
The importance of innate immune cells in HIV-1 pathogenesis and protection has been highlighted by the role of natural killer (NK) cells in the containment of viral replication. Use of peripheral blood mononuclear cells (PBMC) in immunologic studies provides both HIV-1 target cells (ie. CD4+ T cells), as well as anti-HIV-1 effector cells, such as NK cells. In this study, NK and other immune cell populations were analyzed in HIV-negative donor PBMC for an impact on the anti-HIV activity of polyclonal and monoclonal antibodies. NK cell percentages were significantly higher in donor PBMC that supported lower levels of viral replication. While the percentage of NK cells was not directly associated with neutralization titers, NK cell-depletion significantly diminished the antiviral antibody activity by up to three logs, and polymorphisms in NK killer immunoglobulin receptor (KIR) and FcγRIIIa alleles appear to be associated with this affect. These findings demonstrate that NK cells and NK cell receptor polymorphisms may influence assessment of traditional HIV-1 neutralization in a platform where antibody is continuously present. This format appears to simultaneously assess conventional entry inhibition (neutralization) and non-neutralizing antibody-dependent HIV inhibition, which may provide the opportunity to delineate the dominant antibody function(s) in polyclonal vaccine responses.
Conflict of interest statement
Figures

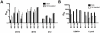
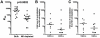

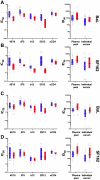

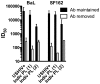
References
-
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. - PubMed
-
- Thongcharoen P, Suriyanon V, Paris RM, Khamboonruang C, de Souza MS, et al. A Phase 1/2 Comparative Vaccine Trial of the Safety and Immunogenicity of a CRF01_AE (Subtype E) Candidate Vaccine: ALVAC-HIV (vCP1521) Prime With Oligomeric gp160 (92TH023/LAI-DID) or Bivalent gp120 (CM235/SF2) Boost. J Acquir Immune Defic Syndr. 2007;46:48–55. - PubMed
-
- Pitisuttithum P, Berman PW, Phonrat B, Suntharasamai P, Raktham S, et al. Phase I/II study of a candidate vaccine designed against the B and E subtypes of HIV-1. J Acquir Immune Defic Syndr. 2004;37:1160–1165. - PubMed
-
- Pitisuttithum P, Nitayaphan S, Thongcharoen P, Khamboonruang C, Kim J, et al. Safety and immunogenicity of combinations of recombinant subtype E and B human immunodeficiency virus type 1 envelope glycoprotein 120 vaccines in healthy Thai adults. J Infect Dis. 2003;188:219–227. - PubMed
-
- Stamatatos L, Morris L, Burton DR, Mascola JR. Neutralizing antibodies generated during natural HIV-1 infection: good news for an HIV-1 vaccine? Nat Med. 2009;15:866–870. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

