Specific roles of Akt iso forms in apoptosis and axon growth regulation in neurons
- PMID: 22509246
- PMCID: PMC3324480
- DOI: 10.1371/journal.pone.0032715
Specific roles of Akt iso forms in apoptosis and axon growth regulation in neurons
Abstract
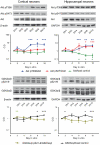
Akt is a member of the AGC kinase family and consists of three isoforms. As one of the major regulators of the class I PI3 kinase pathway, it has a key role in the control of cell metabolism, growth, and survival. Although it has been extensively studied in the nervous system, we have only a faint knowledge of the specific role of each isoform in differentiated neurons. Here, we have used both cortical and hippocampal neuronal cultures to analyse their function. We characterized the expression and function of Akt isoforms, and some of their substrates along different stages of neuronal development using a specific shRNA approach to elucidate the involvement of each isoform in neuron viability, axon development, and cell signalling. Our results suggest that three Akt isoforms show substantial compensation in many processes. However, the disruption of Akt2 and Akt3 significantly reduced neuron viability and axon length. These changes correlated with a tendency to increase in active caspase 3 and a decrease in the phosphorylation of some elements of the mTORC1 pathway. Indeed, the decrease of Akt2 and more evident the inhibition of Akt3 reduced the expression and phosphorylation of S6. All these data indicate that Akt2 and Akt3 specifically regulate some aspects of apoptosis and cell growth in cultured neurons and may contribute to the understanding of mechanisms of neuron death and pathologies that show deregulated growth.
Conflict of interest statement
Figures






References
-
- Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol. 2010;11:9–22. - PubMed
-
- Dummler B, Hemmings BA. Physiological roles of PKB/Akt isoforms in development and disease. Biochem Soc Trans. 2007;35:231–235. - PubMed
-
- Yang ZZ, Tschopp O, Baudry A, Dummler B, Hynx D, et al. Physiological functions of protein kinase B/Akt. Biochem Soc Trans. 2004;32:350–354. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

