Erythropoietin receptor signaling is membrane raft dependent
- PMID: 22509308
- PMCID: PMC3317978
- DOI: 10.1371/journal.pone.0034477
Erythropoietin receptor signaling is membrane raft dependent
Abstract
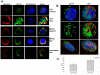
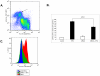
Upon erythropoietin (Epo) engagement, Epo-receptor (R) homodimerizes to activate JAK2 and Lyn, which phosphorylate STAT5. Although recent investigations have identified key negative regulators of Epo-R signaling, little is known about the role of membrane localization in controlling receptor signal fidelity. Here we show a critical role for membrane raft (MR) microdomains in creation of discrete signaling platforms essential for Epo-R signaling. Treatment of UT7 cells with Epo induced MR assembly and coalescence. Confocal microscopy showed that raft aggregates significantly increased after Epo stimulation (mean, 4.3±1.4(SE) vs. 25.6±3.2 aggregates/cell; p≤0.001), accompanied by a >3-fold increase in cluster size (p≤0.001). Raft fraction immunoblotting showed Epo-R translocation to MR after Epo stimulation and was confirmed by fluorescence microscopy in Epo stimulated UT7 cells and primary erythroid bursts. Receptor recruitment into MR was accompanied by incorporation of JAK2, Lyn, and STAT5 and their activated forms. Raft disruption by cholesterol depletion extinguished Epo induced Jak2, STAT5, Akt and MAPK phosphorylation in UT7 cells and erythroid progenitors. Furthermore, inhibition of the Rho GTPases Rac1 or RhoA blocked receptor recruitment into raft fractions, indicating a role for these GTPases in receptor trafficking. These data establish a critical role for MR in recruitment and assembly of Epo-R and signal intermediates into discrete membrane signaling units.
Conflict of interest statement
Figures




References
-
- Witthuhn B, Quelle F, Silvennoinen O, Yi T, Tang B, et al. Jak2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell. 1993;74:227–236. - PubMed
-
- Wojchowski D, Gregory R, Miller C, Pandit A, Pircher T. Signal transduction in the erythropoietin receptor system. Experimental cell research. 1999;253:143–156. - PubMed
-
- Rane SG, Reddy EP. JAKs, STATs and Src kinases in hematopoiesis. Oncogene. 2002;21:3334–3358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

