Efficacy of oral metronidazole with vaginal clindamycin or vaginal probiotic for bacterial vaginosis: randomised placebo-controlled double-blind trial
- PMID: 22509319
- PMCID: PMC3317998
- DOI: 10.1371/journal.pone.0034540
Efficacy of oral metronidazole with vaginal clindamycin or vaginal probiotic for bacterial vaginosis: randomised placebo-controlled double-blind trial
Abstract
Background: To determine if oral metronidazole (MTZ-400 mg bid) with 2% vaginal clindamycin-cream (Clind) or a Lactobacillus acidophilus vaginal-probiotic containing oestriol (Prob) reduces 6-month bacterial vaginosis (BV) recurrence.
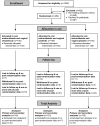
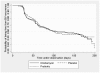
Methods: Double-blind placebo-controlled parallel-group single-site study with balanced randomization (1:1:1) conducted at Melbourne Sexual Health Centre, Australia. Participants with symptomatic BV [Nugent Score (NS) = 7-10 or ≥3 Amsel's criteria and NS = 4-10], were randomly allocated to MTZ-Clind, MTZ-Prob or MTZ-Placebo and assessed at 1,2,3 and 6 months. MTZ and Clind were administered for 7 days and Prob and Placebo for 12 days. Primary outcome was BV recurrence (NS of 7-10) on self-collected vaginal-swabs over 6-months. Cumulative BV recurrence rates were compared between groups by Chi-squared statistics. Kaplan-Meier, log rank and Cox regression analyses were used to compare time until and risk of BV recurrence between groups.
Results: 450 18-50 year old females were randomized and 408 (91%), equally distributed between groups, provided ≥1 NS post-randomization and were included in analyses; 42 (9%) participants with no post-randomization data were excluded. Six-month retention rates were 78% (n = 351). One-month BV recurrence (NS 7-10) rates were 3.6% (5/140), 6.8% (9/133) and 9.6% (13/135) in the MTZ-Clind, MTZ-Prob and MTZ-Placebo groups respectively, p = 0.13. Hazard ratios (HR) for BV recurrence at one-month, adjusted for adherence to vaginal therapy, were 0.43 (95%CI 0.15-1.22) and 0.75 (95% CI 0.32-1.76) in the MTZ-Clind and MTZ-Prob groups compared to MTZ-Plac respectively. Cumulative 6-month BV recurrence was 28.2%; (95%CI 24.0-32.7%) with no difference between groups, p = 0.82; HRs for 6-month BV recurrence for MTZ-Clind and MTZ-Prob compared to MTZ-Plac, adjusted for adherence to vaginal therapy were 1.09(95% CI = 0.70-1.70) and 1.03(95% CI = 0.65-1.63), respectively. No serious adverse events occurred.
Conclusion: Combining the recommended first line therapies of oral metronidazole and vaginal clindamycin, or oral metronidazole with an extended-course of a commercially available vaginal-L.acidophilus probiotic, does not reduce BV recurrence.
Trial registration: ANZCTR.org.au ACTRN12607000350426.
Conflict of interest statement
Figures
References
-
- Allsworth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001–2004 national health and nutrition examination survey data. Obstet Gynecol. 2007;109:114–120. - PubMed
-
- Koumans EH, Markowitz LE, Hogan V. Indications for therapy and treatment recommendations for bacterial vaginosis in nonpregnant and pregnant women: a synthesis of data. Clin Infect Dis. 2002;35:S152–172. - PubMed
-
- Oduyebo OO, Anorlu RI, Ogunsola FT. The effects of antimicrobial therapy on bacterial vaginosis in non-pregnant women. Cochrane Database Syst Rev. 2009:CD006055. - PubMed
-
- Bradshaw CS, Morton AN, Hocking J, Garland SM, Morris MB, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. Journal of Infectious Diseases. 2006;193:1478–1489. - PubMed
-
- Senok AC, Verstraelen H, Temmerman M, Botta GA. Probiotics for the treatment of bacterial vaginosis. Cochrane Database Syst Rev. 2009:CD006289. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous