Gastrin-releasing peptide signaling plays a limited and subtle role in amygdala physiology and aversive memory
- PMID: 22509372
- PMCID: PMC3324554
- DOI: 10.1371/journal.pone.0034963
Gastrin-releasing peptide signaling plays a limited and subtle role in amygdala physiology and aversive memory
Abstract
Links between synaptic plasticity in the lateral amygdala (LA) and Pavlovian fear learning are well established. Neuropeptides including gastrin-releasing peptide (GRP) can modulate LA function. GRP increases inhibition in the LA and mice lacking the GRP receptor (GRPR KO) show more pronounced and persistent fear after single-trial associative learning. Here, we confirmed these initial findings and examined whether they extrapolate to more aspects of amygdala physiology and to other forms of aversive associative learning. GRP application in brain slices from wildtype but not GRPR KO mice increased spontaneous inhibitory activity in LA pyramidal neurons. In amygdala slices from GRPR KO mice, GRP did not increase inhibitory activity. In comparison to wildtype, short- but not long-term plasticity was increased in the cortico-lateral amygdala (LA) pathway of GRPR KO amygdala slices, whereas no changes were detected in the thalamo-LA pathway. In addition, GRPR KO mice showed enhanced fear evoked by single-trial conditioning and reduced spontaneous firing of neurons in the central nucleus of the amygdala (CeA). Altogether, these results are consistent with a potentially important modulatory role of GRP/GRPR signaling in the amygdala. However, administration of GRP or the GRPR antagonist (D-Phe(6), Leu-NHEt(13), des-Met(14))-Bombesin (6-14) did not affect amygdala LTP in brain slices, nor did they affect the expression of conditioned fear following intra-amygdala administration. GRPR KO mice also failed to show differences in fear expression and extinction after multiple-trial fear conditioning, and there were no differences in conditioned taste aversion or gustatory neophobia. Collectively, our data indicate that GRP/GRPR signaling modulates amygdala physiology in a paradigm-specific fashion that likely is insufficient to generate therapeutic effects across amygdala-dependent disorders.
Conflict of interest statement
Figures
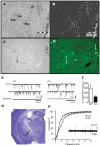
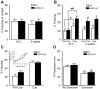


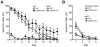
Similar articles
-
Gastrin-releasing peptide regulates fear learning under stressed conditions via activation of the amygdalostriatal transition area.Mol Psychiatry. 2022 Mar;27(3):1694-1703. doi: 10.1038/s41380-021-01408-3. Epub 2022 Jan 8. Mol Psychiatry. 2022. PMID: 34997193
-
Identification of a signaling network in lateral nucleus of amygdala important for inhibiting memory specifically related to learned fear.Cell. 2002 Dec 13;111(6):905-18. doi: 10.1016/s0092-8674(02)01116-9. Cell. 2002. PMID: 12526815
-
Bombesin-like peptide recruits disinhibitory cortical circuits and enhances fear memories.Cell. 2021 Oct 28;184(22):5622-5634.e25. doi: 10.1016/j.cell.2021.09.013. Epub 2021 Oct 4. Cell. 2021. PMID: 34610277 Free PMC article.
-
Gastrin-releasing peptide receptor signaling in the integration of stress and memory.Neurobiol Learn Mem. 2014 Jul;112:44-52. doi: 10.1016/j.nlm.2013.08.013. Epub 2013 Aug 31. Neurobiol Learn Mem. 2014. PMID: 24001571 Review.
-
Gastrin-releasing peptide as a molecular target for inflammatory diseases: an update.Inflamm Allergy Drug Targets. 2013 Jun;12(3):172-7. doi: 10.2174/1871528111312030003. Inflamm Allergy Drug Targets. 2013. PMID: 23621446 Review.
Cited by
-
A Novel Bio-Psychosocial-Behavioral Treatment Model of Panic Disorder.Psychiatry Investig. 2019 Jan;16(1):4-15. doi: 10.30773/pi.2018.08.21.1. Epub 2018 Oct 11. Psychiatry Investig. 2019. PMID: 30301303 Free PMC article.
-
Gastrin-releasing peptide receptors in the central nervous system: role in brain function and as a drug target.Front Endocrinol (Lausanne). 2012 Dec 17;3:159. doi: 10.3389/fendo.2012.00159. eCollection 2012. Front Endocrinol (Lausanne). 2012. PMID: 23251133 Free PMC article.
-
Dopamine release and dopamine-related gene expression in the amygdala are modulated by the gastrin-releasing peptide in opposite directions during stress-enhanced fear learning and extinction.Mol Psychiatry. 2025 Jun;30(6):2381-2394. doi: 10.1038/s41380-024-02843-8. Epub 2024 Nov 23. Mol Psychiatry. 2025. PMID: 39580604 Free PMC article.
-
Gastrin-releasing peptide regulates fear learning under stressed conditions via activation of the amygdalostriatal transition area.Mol Psychiatry. 2022 Mar;27(3):1694-1703. doi: 10.1038/s41380-021-01408-3. Epub 2022 Jan 8. Mol Psychiatry. 2022. PMID: 34997193
-
Self administration of oxycodone by adolescent and adult mice affects striatal neurotransmitter receptor gene expression.Neuroscience. 2014 Jan 31;258:280-91. doi: 10.1016/j.neuroscience.2013.10.062. Epub 2013 Nov 9. Neuroscience. 2014. PMID: 24220688 Free PMC article.
References
-
- Buchel C, Dolan RJ. Classical fear conditioning in functional neuroimaging. Curr Opin Neurobiol. 2000;10:219–223. - PubMed
-
- Delgado MR, Olsson A, Phelps EA. Extending animal models of fear conditioning to humans. Biol Psychol. 2006;73:39–48. - PubMed
-
- Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol Psychol. 2006;73:61–71. - PubMed
-
- Rogan MT, LeDoux JE. Emotion: systems, cells, synaptic plasticity. Cell. 1996;85:469–475. - PubMed
-
- Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev. 1999;23:743–760. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

