Incrimination of Phlebotomus kandelakii and Phlebotomus balcanicus as vectors of Leishmania infantum in Tbilisi, Georgia
- PMID: 22509422
- PMCID: PMC3317916
- DOI: 10.1371/journal.pntd.0001609
Incrimination of Phlebotomus kandelakii and Phlebotomus balcanicus as vectors of Leishmania infantum in Tbilisi, Georgia
Abstract
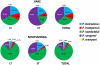
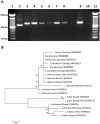
A survey of potential vector sand flies was conducted in the neighboring suburban communities of Vake and Mtatsminda districts in an active focus of visceral Leishmaniasis (VL) in Tbilisi, Georgia. Using light and sticky-paper traps, 1,266 male and 1,179 female sand flies were collected during 2006-2008. Five Phlebotomus species of three subgenera were collected: Phlebotomus balcanicus Theodor and Phlebotomus halepensis Theodor of the subgenus Adlerius; Phlebotomus kandelakii Shchurenkova and Phlebotomus wenyoni Adler and Theodor of the subgenus Larroussius; Phlebotomus sergenti Perfil'ev of the subgenus Paraphlebotomus. Phlebotomus sergenti (35.1%) predominated in Vake, followed by P. kandelakii (33.5%), P. balcanicus (18.9%), P. halepensis (12.2%), and P. wenyoni (0.3%). In Mtatsminda, P. kandelakii (76.8%) comprised over three fourths of collected sand flies, followed by P. sergenti (12.6%), P. balcanicus (5.8%), P. halepensis (3.7%), and P. wenyoni (1.1%). The sand fly season in Georgia is exceptionally short beginning in early June, peaking in July and August, then declining to zero in early September. Of 659 female sand flies examined for Leishmania, 12 (1.8%) specimens without traces of blood were infected including 10 of 535 P. kandelakii (1.9%) and two of 40 P. balcanicus (5.0%). Six isolates were successfully cultured and characterized as Leishmania by PCR. Three isolates from P. kandelakii (2) and P. balcanicus (1) were further identified as L. infantum using sequence alignment of the 70 kDa heat-shock protein gene. Importantly, the sand fly isolates showed a high percent identity (99.8%-99.9%) to human and dog isolates from the same focus, incriminating the two sand fly species as vectors. Blood meal analysis showed that P. kandelakii preferentially feeds on dogs (76%) but also feeds on humans. The abundance, infection rate and feeding behavior of P. kandelakii and the infection rate in P. balcanicus establish these species as vectors in the Tbilisi VL focus.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Lemer M. Some problems of biology of sandflies in the natural nidi of visceral leishmaniasis of Georgia. 1955. pp. 409–414. Sborn Rabot 3 Posvyashch 70 -Let Yubil E N Pavlovskii, Moskva.
-
- Maruashvili G. About types of visceral leishmaniasis foci (Russian). Medical Parasitology (Moscow) 1961;30:188–190. - PubMed
-
- Chubabria G, Zenaishvili O. Modern concepts of visceral leishmaniasis in Georgia (Russian). Medical Parasitology (Moscow) 2003;2:27–30.
-
- Killick-Kendrick R. Phlebotomine vectors of the leishmaniases: a review. Med Vet Entomol. 1990;4:1–24. - PubMed
Publication types
MeSH terms
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

