Design of substituted bis-Tetrahydrofuran (bis-THF)-derived Potent HIV-1 Protease Inhibitors, Protein-ligand X-ray Structure, and Convenient Syntheses of bis-THF and Substituted bis-THF Ligands
- PMID: 22509432
- PMCID: PMC3325757
- DOI: 10.1021/ml100289m
Design of substituted bis-Tetrahydrofuran (bis-THF)-derived Potent HIV-1 Protease Inhibitors, Protein-ligand X-ray Structure, and Convenient Syntheses of bis-THF and Substituted bis-THF Ligands
Abstract
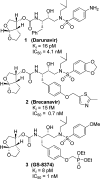
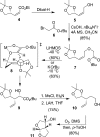
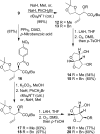
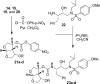
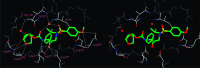
We investigated substituted bis-THF-derived HIV-1 protease inhibitors in order to enhance ligand-binding site interactions in the HIV-1 protease active site. In this context, we have carried out convenient syntheses of optically active bis-THF and C4-substituted bis-THF ligands using a [2,3]-sigmatropic rearrangement as the key step. The synthesis provided convenient access to a number of substituted bis-THF derivatives. Incorporation of these ligands led to a series of potent HIV-1 protease inhibitors. Inhibitor 23c turned out to be the most potent (K(i) = 2.9 pM; IC(50) = 2.4 nM) among the inhibitors. An X-ray structure of 23c-bound HIV-1 protease showed extensive interactions of the inhibitor with the protease active site, including a unique water-mediated hydrogen bond to the Gly-48 amide NH in the S2 site.
Figures
Similar articles
-
Design of gem-difluoro-bis-tetrahydrofuran as P2 ligand for HIV-1 protease inhibitors to improve brain penetration: synthesis, X-ray studies, and biological evaluation.ChemMedChem. 2015 Jan;10(1):107-15. doi: 10.1002/cmdc.201402358. Epub 2014 Oct 21. ChemMedChem. 2015. PMID: 25336073 Free PMC article.
-
Design of HIV protease inhibitors targeting protein backbone: an effective strategy for combating drug resistance.Acc Chem Res. 2008 Jan;41(1):78-86. doi: 10.1021/ar7001232. Epub 2007 Aug 28. Acc Chem Res. 2008. PMID: 17722874 Review.
-
Nonpeptidal P2 ligands for HIV protease inhibitors: structure-based design, synthesis, and biological evaluation.J Med Chem. 1996 Aug 16;39(17):3278-90. doi: 10.1021/jm960128k. J Med Chem. 1996. PMID: 8765511
-
Design of HIV-1 Protease Inhibitors with Amino-bis-tetrahydrofuran Derivatives as P2-Ligands to Enhance Backbone-Binding Interactions: Synthesis, Biological Evaluation, and Protein-Ligand X-ray Studies.J Med Chem. 2015 Sep 10;58(17):6994-7006. doi: 10.1021/acs.jmedchem.5b00900. Epub 2015 Aug 25. J Med Chem. 2015. PMID: 26306007 Free PMC article.
-
Darunavir, a conceptually new HIV-1 protease inhibitor for the treatment of drug-resistant HIV.Bioorg Med Chem. 2007 Dec 15;15(24):7576-80. doi: 10.1016/j.bmc.2007.09.010. Epub 2007 Sep 14. Bioorg Med Chem. 2007. PMID: 17900913 Free PMC article. Review.
Cited by
-
GRL-079, a Novel HIV-1 Protease Inhibitor, Is Extremely Potent against Multidrug-Resistant HIV-1 Variants and Has a High Genetic Barrier against the Emergence of Resistant Variants.Antimicrob Agents Chemother. 2018 Apr 26;62(5):e02060-17. doi: 10.1128/AAC.02060-17. Print 2018 May. Antimicrob Agents Chemother. 2018. PMID: 29463535 Free PMC article.
-
Estimation of genetic diversity and population structure in Tinospora cordifolia using SSR markers.3 Biotech. 2020 Jul;10(7):310. doi: 10.1007/s13205-020-02300-7. Epub 2020 Jun 16. 3 Biotech. 2020. PMID: 32582507 Free PMC article.
-
SAR and Lead Optimization of an HIV-1 Vif-APOBEC3G Axis Inhibitor.ACS Med Chem Lett. 2012 Jun 14;3(6):465-469. doi: 10.1021/ml300037k. ACS Med Chem Lett. 2012. PMID: 24533175 Free PMC article.
-
Highly resistant HIV-1 proteases and strategies for their inhibition.Future Med Chem. 2015;7(8):1023-38. doi: 10.4155/fmc.15.44. Future Med Chem. 2015. PMID: 26062399 Free PMC article. Review.
-
Investigational protease inhibitors as antiretroviral therapies.Expert Opin Investig Drugs. 2016 Oct;25(10):1189-200. doi: 10.1080/13543784.2016.1212837. Epub 2016 Aug 2. Expert Opin Investig Drugs. 2016. PMID: 27415449 Free PMC article. Review.
References
-
- FDA approves Darunavir on June 23, 2006: FDA approved a new HIV treatment for patients who do not respond to existing drugs. Please see http://www.fda.gov/bbs/topics/NEWS/2006/NEW01395.html.
-
-
On October 21, 2008, FDA granted traditional approval to Prezista (darunavir), coadministered with ritonavir and with other antiretroviral agents, for the treatment of HIV-1 infection in treatment-experienced adult patients. In addition, a new dosing regimen for treatment-naïve adult patients was approved.
-
-
- Ghosh A. K.; Sridhar P. R.; Kumaragurubaran N.; Koh Y.; Weber I. T.; Mitsuya H. A Privileged Ligand for Darunavir and a New Generation of HIV-Protease Inhibitors That Combat Drug-Resistance. ChemMedChem 2006, 1, 939–950. - PubMed
-
- Ghosh A. K.; Chapsal B. D.; Weber I. T.; Mitsuya H. Design of HIV Protease Inhibitors Targeting Protein Backbone: An Effective Strategy for Combating Drug Resistance. Acc. Chem. Res. 2008, 41, 78–86. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

