Human ABCG2: structure, function, and its role in multidrug resistance
- PMID: 22509477
- PMCID: PMC3325772
Human ABCG2: structure, function, and its role in multidrug resistance
Abstract
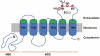
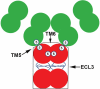
Human ABCG2 is a member of the ATP-binding cassette (ABC) transporter superfamily and is known to contribute to multidrug resistance (MDR) in cancer chemotherapy. Among ABC transporters that are known to cause MDR, ABCG2 is particularly interesting for its potential role in protecting cancer stem cells and its complex oligomeric structure. Recent studies have also revealed that the biogenesis of ABCG2 could be modulated by small molecule compounds. These modulators, upon binding to ABCG2, accelerate the endocytosis and trafficking to lysosome for degradation and effectively reduce the half-life of ABCG2. Hence, targeting ABCG2 stability could be a new venue for therapeutic discovery to sensitize drug resistant human cancers. In this report, we review recent progress on understanding the structure, function, biogenesis, as well as physiological and pathophysiological functions of ABCG2.
Keywords: ATP-binding cassette; Human ABCG2; cancer; chemotherapy; function; multidrug resistance; structure.
Figures
References
-
- Boesen JJ, Niericker MJ, Dieteren N, Simons JW. How variable is a spontaneous mutation rate in cultured mammalian cells? Mutat Res. 1994;307:121–129. - PubMed
-
- Zhang JT. Use of arrays to investigate the contribution of ATP-binding cassette transporters to drug resistance in cancer chemotherapy and prediction of chemosensitivity. Cell Res. 2007;17:311–323. - PubMed
-
- Xu J, Peng H, Zhang JT. Human multidrug transporter ABCG2, a target for sensitizing drug resistance in cancer chemotherapy. Curr Med Chem. 2007;14:689–701. - PubMed
-
- Zhang JT. Biochemistry and pharmacology of the human multidrug resistance gene product, ABCG2. J. Cent. South Univ. (Med Sci) 2007;32:531–541. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
