Optically stable biocompatible flame-made SiO2-coated Y2O3:Tb3+ nanophosphors for cell imaging
- PMID: 22509739
- PMCID: PMC3717410
- DOI: 10.1021/nn205035p
Optically stable biocompatible flame-made SiO2-coated Y2O3:Tb3+ nanophosphors for cell imaging
Abstract
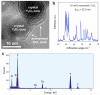
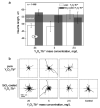
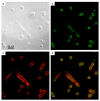
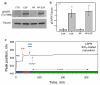
Nanophosphors are light-emitting materials with stable optical properties that represent promising tools for bioimaging. The synthesis of nanophosphors, and thus the control of their surface properties, is, however, challenging. Here, flame aerosol technology is exploited to generate Tb-activated Y(2)O(3) nanophosphors (∼25 nm) encapsulated in situ by a nanothin amorphous inert SiO(2) film. The nanocrystalline core exhibits a bright green luminescence following the Tb(3+) ion transitions, while the hermetic SiO(2)-coating prevents any unspecific interference with cellular activities. The SiO(2)-coated nanophosphors display minimal photobleaching upon imaging and can be easily functionalized through surface absorption of biological molecules. Therefore, they can be used as bionanoprobes for cell detection and for long-term monitoring of cellular activities. As an example, we report on the interaction between epidermal growth factor (EGF)-functionalized nanophosphors and mouse melanoma cells. The cellular uptake of the nanophosphors is visualized with confocal microscopy, and the specific activation of EGF receptors is revealed with biochemical techniques. Altogether, our results establish SiO(2)-coated Tb-activated Y(2)O(3) nanophosphors as superior imaging tools for biological applications.
Figures



Similar articles
-
Bio-mediated route for the synthesis of shape tunable Y₂O₃: Tb³⁺ nanoparticles: Photoluminescence and antibacterial properties.Spectrochim Acta A Mol Biomol Spectrosc. 2015;151:131-40. doi: 10.1016/j.saa.2015.06.081. Epub 2015 Jun 23. Spectrochim Acta A Mol Biomol Spectrosc. 2015. PMID: 26125993
-
Aqueous dispersible green luminescent yttrium oxide:terbium microspheres with nanosilica shell coating.Spectrochim Acta A Mol Biomol Spectrosc. 2019 Mar 15;211:348-355. doi: 10.1016/j.saa.2018.12.015. Epub 2018 Dec 11. Spectrochim Acta A Mol Biomol Spectrosc. 2019. PMID: 30583166
-
Color-tunable nanophosphors by co-doping flame-made Y2O3 with Tb and Eu.J Phys Chem C Nanomater Interfaces. 2011 Feb 3;115(4):1084-1089. doi: 10.1021/jp106137u. J Phys Chem C Nanomater Interfaces. 2011. PMID: 23730401 Free PMC article.
-
Green, Silica-Coated Monoclinic Y(2)O(3):Tb(3+) Nanophosphors: Flame Synthesis and Characterization.J Phys Chem C Nanomater Interfaces. 2012 Feb 23;116(7):4493-4499. doi: 10.1021/jp211722z. J Phys Chem C Nanomater Interfaces. 2012. PMID: 23408153 Free PMC article.
-
Nanophosphors-Based White Light Sources.Nanomaterials (Basel). 2019 Jul 22;9(7):1048. doi: 10.3390/nano9071048. Nanomaterials (Basel). 2019. PMID: 31336578 Free PMC article. Review.
Cited by
-
Synergistic Effect of SiO2 and Fe3O4 Nanoparticles in Autophagy Modulation.Nanomaterials (Basel). 2024 Jun 15;14(12):1033. doi: 10.3390/nano14121033. Nanomaterials (Basel). 2024. PMID: 38921909 Free PMC article.
-
Toward Contactless Biology: Acoustophoretic DNA Transfection.Sci Rep. 2016 Feb 1;6:20023. doi: 10.1038/srep20023. Sci Rep. 2016. PMID: 26828312 Free PMC article.
-
Systemic antitumor immune response of doped yttria nanoscintillators under low-dose x-ray irradiation.Sci Adv. 2025 Mar 28;11(13):eadr4008. doi: 10.1126/sciadv.adr4008. Epub 2025 Mar 26. Sci Adv. 2025. PMID: 40138411 Free PMC article.
-
Near-infrared excited luminescence and in vitro imaging of HeLa cells by using Mn2+ enhanced Tb3+ and Yb3+ cooperative upconversion in NaYF4 nanocrystals.Nanoscale Adv. 2019 Jul 12;1(9):3463-3473. doi: 10.1039/c9na00336c. eCollection 2019 Sep 11. Nanoscale Adv. 2019. PMID: 36133550 Free PMC article.
-
Tracking translocation of industrially relevant engineered nanomaterials (ENMs) across alveolar epithelial monolayers in vitro.Nanotoxicology. 2014 Aug;8 Suppl 1(0 1):216-25. doi: 10.3109/17435390.2013.879612. Epub 2014 Jan 30. Nanotoxicology. 2014. PMID: 24479615 Free PMC article.
References
-
- Project on Emerging Nanotechnologies. 2011 Oct; www.nanotechproject.org.
-
- Alivisatos P. The Use of Nanocrystals in Biological Detection. Nature Biotechnol. 2004;22:47–52. - PubMed
-
- Norris DJ, Efros AL, Erwin SC. Doped Nanocrystals. Science. 2008;319:1776–1779. - PubMed
-
- Nirmal M, Dabbousi BO, Bawendi MG, Macklin JJ, Trautman JK, Harris TD, Brus LE. Fluorescence Intermittency in Single Cadmium Selenide Nanocrystals. Nature. 1996;383:802–804.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

