Model IgG monoclonal autoantibody-anti-idiotype pair for dissecting the humoral immune response to oxidized low density lipoprotein
- PMID: 22509912
- PMCID: PMC3326269
- DOI: 10.1089/hyb.2011.0058
Model IgG monoclonal autoantibody-anti-idiotype pair for dissecting the humoral immune response to oxidized low density lipoprotein
Abstract
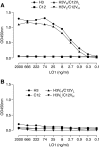
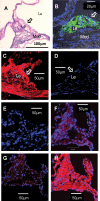
Increasing evidence implicates IgG autoantibodies against oxidized forms of low density lipoprotein (oxLDL) in the pathophysiology of atherosclerotic arterial disease. However, insufficient knowledge of their structure and function is a key gap. Using an elderly LDL receptor-deficient atherosclerotic mouse, we isolated a novel IgG3k against oxLDL (designated MAb LO1). LO1 reacts with copper-oxidized LDL, but minimally with native LDL. Further analysis showed that MAb LO1 also reacts in vitro with malondialdehyde-conjugated LDL (MDA-LDL), a known key epitope in copper-oxidized LDL preparations. By screening a phage library expressing single chain variable region antibodies (scFv), we selected an anti-idiotype scFv (designated H3) that neutralizes MAb LO1 binding to MDA-LDL. Amino acid substitutions between H3 and an irrelevant control scFv C12 showed that residues in the H3 CDRH2, CDRH3, and CDRL2 are all critical for MAb LO1 binding, consistent with a conformational epitope on H3 involving both heavy and light chains. Comparison of amino acids in H3 CDRH2 and CDRL2 with apoB, the major LDL protein, showed homologous sequences, suggesting H3 has structural similarities to the MAb LO1 binding site on MDA-LDL. Immunocytochemical staining showed that MAb LO1 binds epitopes in mouse and human atherosclerotic lesions. The MAb LO1-H3 combination therefore provides a very promising model for analyzing the structure and function of an individual IgG autoantibody in relation to atherosclerosis.
Figures



Similar articles
-
Characterization of human monoclonal autoantibody Fab fragments against oxidized LDL.Mol Immunol. 2007 Feb;44(5):827-36. doi: 10.1016/j.molimm.2006.04.005. Epub 2006 Jun 21. Mol Immunol. 2007. PMID: 16793138
-
Near Infrared Fluorescence (NIRF) Molecular Imaging of Oxidized LDL with an Autoantibody in Experimental Atherosclerosis.Sci Rep. 2016 Feb 25;6:21785. doi: 10.1038/srep21785. Sci Rep. 2016. PMID: 26911995 Free PMC article.
-
Human monoclonal Fab and human plasma antibodies to carbamyl-epitopes cross-react with malondialdehyde-adducts.Immunology. 2014 Mar;141(3):416-30. doi: 10.1111/imm.12204. Immunology. 2014. PMID: 24168430 Free PMC article.
-
Antibodies to oxidized low density lipoprotein: epidemiological studies and potential clinical applications in cardiovascular disease.Minerva Cardioangiol. 2007 Dec;55(6):821-37. Minerva Cardioangiol. 2007. PMID: 18091649 Review.
-
Oxidative modification of low-density lipoprotein and immune regulation of atherosclerosis.Prog Lipid Res. 2006 Nov;45(6):466-86. doi: 10.1016/j.plipres.2006.05.001. Epub 2006 May 30. Prog Lipid Res. 2006. PMID: 16790279 Review.
Cited by
-
Oxidised LDL and Anti-Oxidised LDL Antibodies Are Reduced by Lipoprotein Apheresis in a Randomised Controlled Trial on Patients with Refractory Angina and Elevated Lipoprotein(a).Antioxidants (Basel). 2021 Jan 18;10(1):132. doi: 10.3390/antiox10010132. Antioxidants (Basel). 2021. PMID: 33477712 Free PMC article.
-
High Serum Immunoglobulin G and M Levels Predict Freedom From Adverse Cardiovascular Events in Hypertension: A Nested Case-Control Substudy of the Anglo-Scandinavian Cardiac Outcomes Trial.EBioMedicine. 2016 Jul;9:372-380. doi: 10.1016/j.ebiom.2016.06.012. Epub 2016 Jun 20. EBioMedicine. 2016. PMID: 27333022 Free PMC article. Clinical Trial.
-
The Placebo-Controlled Effect of Percutaneous Coronary Intervention on Exercise Induced Changes in Anti-Malondialdehyde-LDL Antibody Levels in Stable Coronary Artery Disease: A Substudy of the ORBITA Trial.Front Cardiovasc Med. 2021 Oct 11;8:757030. doi: 10.3389/fcvm.2021.757030. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34708098 Free PMC article.
-
IgM anti-malondialdehyde low density lipoprotein antibody levels indicate coronary heart disease and necrotic core characteristics in the Nordic Diltiazem (NORDIL) study and the Integrated Imaging and Biomarker Study 3 (IBIS-3).EBioMedicine. 2018 Oct;36:63-72. doi: 10.1016/j.ebiom.2018.08.023. Epub 2018 Aug 18. EBioMedicine. 2018. PMID: 30131305 Free PMC article.
-
A Novel Immunoassay for Malondialdehyde-Conjugated Low-Density Lipoprotein Measures Dynamic Changes in the Blood of Patients Undergoing Coronary Artery Bypass Graft Surgery.Antioxidants (Basel). 2021 Aug 17;10(8):1298. doi: 10.3390/antiox10081298. Antioxidants (Basel). 2021. PMID: 34439546 Free PMC article.
References
-
- Steinberg D. Thematic review series: the pathogenesis of atherosclerosis: an interpretive history of the cholesterol controversy, part III: mechanistically defining the role of hyperlipidemia. J Lipid Res. 2005;46(10):2037–2051. - PubMed
-
- Hansson GK. Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6(7):508–519. - PubMed
-
- Pluddemann A. Neyen C. Gordon S. Macrophage scavenger receptors and host-derived ligands. Methods. 2007;43(3):207–217. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

