Patient-oriented simulation based on Monte Carlo algorithm by using MRI data
- PMID: 22510474
- PMCID: PMC3355000
- DOI: 10.1186/1475-925X-11-21
Patient-oriented simulation based on Monte Carlo algorithm by using MRI data
Abstract
Background: Although Monte Carlo simulations of light propagation in full segmented three-dimensional MRI based anatomical models of the human head have been reported in many articles. To our knowledge, there is no patient-oriented simulation for individualized calibration with NIRS measurement. Thus, we offer an approach for brain modeling based on image segmentation process with in vivo MRI T1 three-dimensional image to investigate the individualized calibration for NIRS measurement with Monte Carlo simulation.
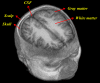
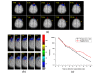
Methods: In this study, an individualized brain is modeled based on in vivo MRI 3D image as five layers structure. The behavior of photon migration was studied for this individualized brain detections based on three-dimensional time-resolved Monte Carlo algorithm. During the Monte Carlo iteration, all photon paths were traced with various source-detector separations for characterization of brain structure to provide helpful information for individualized design of NIRS system.
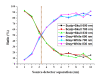
Results: Our results indicate that the patient-oriented simulation can provide significant characteristics on the optimal choice of source-detector separation within 3.3 cm of individualized design in this case. Significant distortions were observed around the cerebral cortex folding. The spatial sensitivity profile penetrated deeper to the brain in the case of expanded CSF. This finding suggests that the optical method may provide not only functional signal from brain activation but also structural information of brain atrophy with the expanded CSF layer. The proposed modeling method also provides multi-wavelength for NIRS simulation to approach the practical NIRS measurement.
Conclusions: In this study, the three-dimensional time-resolved brain modeling method approaches the realistic human brain that provides useful information for NIRS systematic design and calibration for individualized case with prior MRI data.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

