CD36 is involved in astrocyte activation and astroglial scar formation
- PMID: 22510603
- PMCID: PMC3421096
- DOI: 10.1038/jcbfm.2012.52
CD36 is involved in astrocyte activation and astroglial scar formation
Abstract
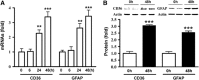
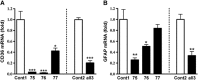
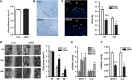
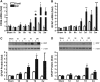
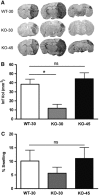
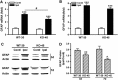
Inflammation is an essential component for glial scar formation. However, the upstream mediator(s) that triggers the process has not been identified. Previously, we showed that the expression of CD36, an inflammatory mediator, occurs in a subset of astcotyes in the peri-infarct area where the glial scar forms. This study investigates a role for CD36 in astrocyte activation and glial scar formation in stroke. We observed that the expression of CD36 and glial fibrillary acidic protein (GFAP) coincided in control and injured astrocytes and in the brain. Furthermore, GFAP expression was attenuated in CD36 small interfering RNA transfected astrocytes or in the brain of CD36 knockout (KO) mice, suggesting its involvement in GFAP expression. Using an in-vitro model of wound healing, we found that CD36 deficiency attenuated the proliferation of astrocytes and delayed closure of the wound gap. Furthermore, stroke-induced GFAP expression and scar formation were significantly attenuated in the CD36 KO mice compared with wild type. These findings identify CD36 as a novel mediator for injury-induced astrogliosis and scar formation. Targeting CD36 may serve as a potential strategy to reduce glial scar formation in stroke.
Figures
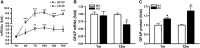

References
-
- Askalan R, Deveber G, Ho M, Ma J, Hawkins C. Astrocytic-inducible nitric oxide synthase in the ischemic developing human brain. Pediatr Res. 2006;60:687–692. - PubMed
-
- Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN, Mucke L, Johnson MH, Sofroniew MV. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23:297–308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

