Entrainment of peripheral clock genes by cortisol
- PMID: 22510707
- PMCID: PMC3426436
- DOI: 10.1152/physiolgenomics.00001.2012
Entrainment of peripheral clock genes by cortisol
Abstract
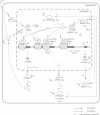
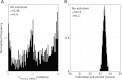
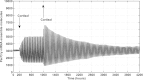
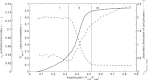
Circadian rhythmicity in mammals is primarily driven by the suprachiasmatic nucleus (SCN), often called the central pacemaker, which converts the photic information of light and dark cycles into neuronal and hormonal signals in the periphery of the body. Cells of peripheral tissues respond to these centrally mediated cues by adjusting their molecular function to optimize organism performance. Numerous systemic cues orchestrate peripheral rhythmicity, such as feeding, body temperature, the autonomic nervous system, and hormones. We propose a semimechanistic model for the entrainment of peripheral clock genes by cortisol as a representative entrainer of peripheral cells. This model demonstrates the importance of entrainer's characteristics in terms of the synchronization and entrainment of peripheral clock genes, and predicts the loss of intercellular synchrony when cortisol moves out of its homeostatic amplitude and frequency range, as has been observed clinically in chronic stress and cancer. The model also predicts a dynamic regime of entrainment, when cortisol has a slightly decreased amplitude rhythm, where individual clock genes remain relatively synchronized among themselves but are phase shifted in relation to the entrainer. The model illustrates how the loss of communication between the SCN and peripheral tissues could result in desynchronization of peripheral clocks.
Figures
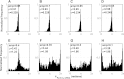

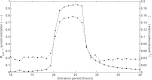

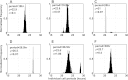
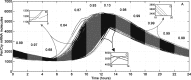
References
-
- An G. In silico experiments of existing and hypothetical cytokine-directed clinical trials using agent-based modeling. Crit Care Med 32: 2050–2060, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

