Safety and efficacy of brentuximab vedotin for Hodgkin lymphoma recurring after allogeneic stem cell transplantation
- PMID: 22510871
- PMCID: PMC3731651
- DOI: 10.1182/blood-2011-12-397893
Safety and efficacy of brentuximab vedotin for Hodgkin lymphoma recurring after allogeneic stem cell transplantation
Abstract
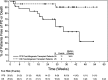
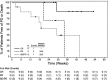
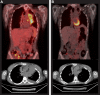
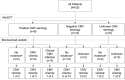
Hodgkin lymphoma (HL) relapsing after allogeneic stem cell transplantation (alloSCT) presents a major clinical challenge. In the present investigation, we evaluated brentuximab vedotin, a CD30-directed Ab-drug conjugate, in 25 HL patients (median age, 32 years; range, 20-56) with recurrent disease after alloSCT (11 unrelated donors). Patients were > 100 days after alloSCT, had no active GVHD, and received a median of 9 (range, 5-19) prior regimens. Nineteen (76%) had refractory disease immediately before enrollment. Patients received 1.2 or 1.8 mg/kg of brentuximab vedotin IV every 3 weeks (median, 8 cycles; range, 1-16). Overall and complete response rates were 50% and 38%, respectively, among 24 evaluable patients. Median time to response was 8.1 weeks, median progression-free survival was 7.8 months, and the median overall survival was not reached. Cough, fatigue, and pyrexia (52% each), nausea and peripheral sensory neuropathy (48% each), and dyspnea (40%) were the most frequent adverse events. The most common adverse events ≥ grade 3 were neutropenia (24%), anemia (20%), thrombocytopenia (16%), and hyperglycemia (12%). Cytomegalovirus was detected in 5 patients (potentially clinically significant in 1). These results support the potential utility of brentuximab vedotin for selected patients with HL relapsing after alloSCT.
Trial registration: ClinicalTrials.gov NCT00947856 NCT01026233 NCT01026415.
Figures
References
-
- Canellos GP, Anderson JR, Propert KJ, et al. Chemotherapy of advanced Hodgkin's disease with MOPP, ABVD, or MOPP alternating with ABVD. N Engl J Med. 1992;327(21):1478–1484. - PubMed
-
- Diehl V, Franklin J, Pfreundschuh M, et al. Standard and increased-dose BEACOPP chemotherapy compared with COPP-ABVD for advanced Hodgkin's disease. N Engl J Med. 2003;348(24):2386–2395. - PubMed
-
- Josting A, Franklin J, May M, et al. New prognostic score based on treatment outcome of patients with relapsed Hodgkin's lymphoma registered in the database of the German Hodgkin's lymphoma study group. J Clin Oncol. 2002;20(1):221–230. - PubMed
-
- Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin's disease: a randomised trial. Lancet. 2002;359(9323):2065–2071. - PubMed
-
- Tarella C, Cuttica A, Vitolo U, et al. High-dose sequential chemotherapy and peripheral blood progenitor cell autografting in patients with refractory and/or recurrent Hodgkin lymphoma: a multicenter study of the intergruppo Italiano Linfomi showing prolonged disease free survival in patients treated at first recurrence. Cancer. 2003;97(11):2748–2759. - PubMed

