The novel intestinal filament organizer IFO-1 contributes to epithelial integrity in concert with ERM-1 and DLG-1
- PMID: 22510987
- PMCID: PMC4074299
- DOI: 10.1242/dev.075788
The novel intestinal filament organizer IFO-1 contributes to epithelial integrity in concert with ERM-1 and DLG-1
Abstract
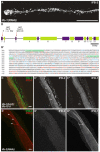
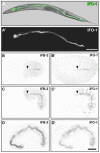
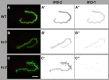
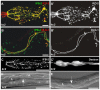
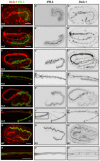
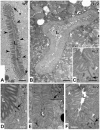
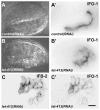
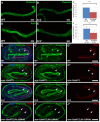
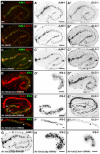
The nematode Caenorhabditis elegans is an excellent model system in which to study in vivo organization and function of the intermediate filament (IF) system for epithelial development and function. Using a transgenic ifb-2::cfp reporter strain, a mutagenesis screen was performed to identify mutants with aberrant expression patterns of the IF protein IFB-2, which is expressed in a dense network at the subapical endotube just below the microvillar brush border of intestinal cells. Two of the isolated alleles (kc2 and kc3) were mapped to the same gene, which we refer to as ifo-1 (intestinal filament organizer). The encoded polypeptide colocalizes with IF proteins and F-actin in the intestine. The apical localization of IFO-1 does not rely on IFB-2 but is dependent on LET-413, a basolateral protein involved in apical junction assembly and maintenance of cell polarity. In mutant worms, IFB-2 and IFC-2 are mislocalized in cytoplasmic granules and accumulate in large aggregates at the C. elegans apical junction (CeAJ) in a DLG-1-dependent fashion. Electron microscopy reveals loss of the prominent endotube and disordered but still intact microvilli. Semiquantitative fluorescence microscopy revealed a significant decrease of F-actin, suggesting a general role of IFO-1 in cytoskeletal organization. Furthermore, downregulation of the cytoskeletal organizer ERM-1 and the adherens junction component DLG-1, each of which leads to F-actin reduction on its own, induces a novel synthetic phenotype in ifo-1 mutants resulting in disruption of the lumen. We conclude that IFO-1 is a multipurpose linker between different cytoskeletal components of the C. elegans intestinal terminal web and contributes to proper epithelial tube formation.
Figures

References
-
- Bement W. M., Mooseker M. S. (1996). The cytoskeleton of the intestinal epithelium: Components, assembly, and dynamic rearrangements. In The Cytoskeleton: A Multi-Volume Treatise, Vol. 3 (ed. John E. H., Ian F. P.), pp. 359–404 Greenwich, CT: JAI Press;
-
- Bosher J. M., Hahn B. S., Legouis R., Sookhareea S., Weimer R. M., Gansmuller A., Chisholm A. D., Rose A. M., Bessereau J. L., Labouesse M. (2003). The Caenorhabditis elegans vab-10 spectraplakin isoforms protect the epidermis against internal and external forces. J. Cell Biol. 161, 757–768 - PMC - PubMed
-
- Bossinger O., Klebes A., Segbert C., Theres C., Knust E. (2001). Zonula adherens formation in Caenorhabditis elegans requires dlg-1, the homologue of the Drosophila gene discs large. Dev. Biol. 230, 29–42 - PubMed
-
- Bossinger O., Fukushige T., Claeys M., Borgonie G., McGhee J. D. (2004). The apical disposition of the Caenorhabditis elegans intestinal terminal web is maintained by LET-413. Dev Biol. 268, 448–456 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

